PharmaCare Policy Manual (Unified)
This web page presents the PharmaCare Policy Manual in its entirety. You can find what you need using the table of contents below, or press CTRL+F to search for keywords. If you would like to view the manual in separate sections, please refer to this page.
As of January 2, 2026:
- The maximum amount PharmaCare will pay per dispense is increasing from $10 to $11
- The maximum number of dispensing fees PharmaCare will pay per day is decreasing, from 3 to 2 for clients receiving daily dispensing
For more details, refer to the December 2025 PharmaCare Newsletter.
For the mandate and scope of the Prosthetic and Orthotic Program, see the P&O Policy Manual.

Table of Contents
Preface to BC PharmaCare Policy Manual
1 Introduction to BC PharmaCare
2 Provider Enrolment in PharmaCare
- Section 2.1 PharmaCare Enrolment
- Section 2.2 Non-Pharmaceutical Supplier Participation Agreement (Removed)1
- Section 2.3 What is PharmaNet?
- Section 2.4 Connecting to PharmaNet
- Section 2.5 Changes to PharmaNet Connections
- Section 2.6 Dealing with PharmaNet Network Outages
- Section 2.7 Fan-Out Messages
1Non-pharmaceutical suppliers now enrol as device providers under the Provider Regulation.
3 Claims Submission
For response and intervention codes, see Appendix A and Appendix B.
- Section 3.1 Which Transactions to Submit on PharmaNet
- Section 3.2 Patients: Identification
- Section 3.3 Patients: Personal Health Numbers
- Section 3.4 Patients: Other Payers
- Section 3.5 Patients: Restricted Claimant Program
- Section 3.6 Patients: With Out-of-Province and Out-of-Country Prescriptions
- Section 3.7 Medical Practitioners: Prescriber IDs & Practitioner Reference IDs
- Section 3.8 (Removed)
- Section 3.9 Medical Practitioners: Authorized Prescribing
- Section 3.10 Medical Practitioners: Practicing Status and Practitioner Restrictions
- Section 3.11 Medical Practitioners: BC Prescription Review Program
- Section 3.12 Drug and Product Identification Numbers
- Section 3.13 Correct Quantities
- Section 3.14 Drug Use Evaluation (DUE)
- Section 3.15 Drug Monograph Information
- Section 3.16 Claim Reversals
- Section 3.17 Prescription Discontinuations
- Section 3.18 Claims for Drug Cost Exceeding $9,999.99
- Section 3.19 Recording Adverse Drug Reaction and Allergy Information in PharmaNet
- Section 3.20 Veterinary prescriptions
- Section 3.21 Office-Use Medications
4 Offline (Manual) Claims
5 Pricing Policies & Product Reimbursement
- Section 5.1 Maximum Days Supply Policy
- Section 5.2 Refilling Prescriptions Too Soon Policy
- Section 5.3 Refilling Prescriptions on the Same Day Policy
- Section 5.4 Travel Supply Policy
- Section 5.5 Correct Quantities Policy
- Section 5.6 Maximum Pricing Policy
- Section 5.7 Actual Acquisition Cost Policy
- Section 5.8 High-Cost Drugs Policy
- Section 5.9 Retail Pricing Policy
- Section 5.10 Full Payment Policy
- Section 5.11 Low Cost Alternative Program
- Section 5.12 Reference Drug Program (RDP)
- Section 5.13 Compounded Prescriptions
- Section 5.14 Insulin
- Section 5.15 Needles and Syringes
- Section 5.16 Blood Glucose Testing
- Section 5.17 Insulin Pumps
- Section 5.18 Insulin Pump Supplies
- Section 5.19 Reimbursement for Non-Returnable High-cost Injectable Drugs
- Section 5.20 Smoking Cessation Program Policy
- Section 5.21 Ostomy Supplies
- Section 5.22 Prosthetics and Orthotics
- Section 5.23 Pricing Exceptions Where Multiple Dosage Forms or Strengths Are Available
- Section 5.24 Drug Shortages
6 Understanding PharmaCare Benefit Status
- Section 6.1 Benefit Status Types
- Section 6.2 Health Canada’s Special Access Program Drugs
- Section 6.3 Special Authority Coverage
- Section 6.4 Collaborative Prescribing Agreements
- Section 6.5 Drug Review Process
7 Coverage Plans
- Section 7.1 Plans Overview
- Section 7.2 Fair PharmaCare
- Section 7.3 Long-term Care (Plan B)
- Section 7.4 Income Assistance (Plan C)
- Section 7.5 Cystic Fibrosis (Plan D)
- Section 7.6 Children in the At Home Program (Plan F)
- Section 7.7 Psychiatric Medications (Plan G)
- Section 7.8 Palliative Care (Plan P)
- Section 7.9 Medication Management (Plan M)
- Section 7.10 Smoking Cessation Program (Plan S)
- Section 7.11 British Columbia Centre for Excellence in HIV/AIDs (Plan X)
- Section 7.12 First Nations Health Benefits (Plan W)
- Section 7.13 Assurance (Plan Z)
8 Pharmacy Fees & Subsidies & Provider Payment
- Section 8.1 About Pharmacy Fees and Subsidies
- Section 8.2 Dispensing Fees
- Section 8.3 Frequency of Dispensing Fee Limits
- Section 8.4 Clinical Services Fees
- Section 8.5 Special Services Fees
- Section 8.6 Trial Prescription Program
- Section 8.7 Capitation Fees for Plan B (Long-term Care)
- Section 8.8 Methadone Maintenance Payment Program
- Section 8.9 Medication Review Services
- Section 8.10 Pharmacist Administration of Drugs and Vaccines
- Section 8.11 Rural Incentive Program
- Section 8.12 Payments to Providers
- Section 8.13 Patient Support Fees
- Section 8.14 Minor Ailments and Contraception Service
- Section 8.15 Rapid Antigen Test Kit Distribution
- Section 8.16 Prescribed Alternatives Witnessing Fee
9 Privacy
- Section 9.1 Identifying Patients
- Section 9.2 PharmaNet user enrolment and site registration in PRIME
- Section 9.3 Access to Patient Information in PharmaNet
- Section 9.4 (Removed)
- Section 9.5 Patient Records
- Section 9.6 Protective Words
- Section 9.7 PharmaNet Security
10 Audit
11 Contacts for Practitioners & Providers
Appendices
- Appendix A Response Codes—PharmaNet adjudication response codes
- Appendix B Intervention Codes—PharmaNet intervention and exception codes
- Appendix C Reference Codes—Practitioner ID reference codes for Canadian prescribers

All information in this policy manual is subject to change in accordance with the provisions of the Pharmaceutical Services Act; the Provider Regulation; the Drug Plans Regulation; the Information Management Regulation; and the Drug Price Regulation. If there is a discrepancy between the legislation/regulations and the information in this manual or on a BC PharmaCare web page, the legislation/regulations prevails.
Equitable coverage
BC PharmaCare is available for any B.C. resident as defined below, according to the rules of their coverage plan.
PharmaCare providers must provide PharmaCare coverage, services and information without discrimination, or intent to discriminate, against a person or group or class of persons because of Indigenous identity, race, colour, ancestry, place of origin, religion, marital status, family status, physical or mental disability, sex, sexual orientation, gender identity or expression, or age, as per the BC Human Rights Code.
Cultural safety and humility
BC PharmaCare counts on pharmacy and device providers to practice cultural safety and humility.
To learn more, read Coming Together for Wellness (PDF, 894KB), a series of articles by First Nations Health Authority (FNHA) and PharmaCare, and consider taking the online San’yas Indigenous Cultural Safety course.
Transparency
BC PharmaCare providers are expected to let clients know when BC PharmaCare is covering medications and providing a fee for services such as a Minor Ailments and Contraception Service (MACS), medication review, vaccination, drug administration, prescription adaptation or renewal, travel supply or emergency supply.
Advertisements for such services should include reference to the B.C. government or to BC PharmaCare. In no circumstance should a pharmacy suggest that such services are provided as a courtesy or favour of the pharmacy.
Posters and brochures for clients
PharmaCare providers are expected to distribute PharmaCare information sheets and post the Fair PharmaCare How to Register and information QR matrix for the public:
- Find the PharmaCare information sheet you need (PDF, 72.6KB)
- Fair PharmaCare how to register (PDF, 75.5KB)
- PharmaCare information sheets – available in 15 languages
Posters for staff
Providers are encouraged to post the following resources for their staff:
- BC PharmaCare resources for pharmacy staff (PDF, 64.5KB)
- PharmaCare Help Desk and Medical Practitioners Line (PDF, 75.5KB)
- How to Claim PharmaCare fees for pharmacy services (PDF, 90.4KB)
PharmaCare Newsletter
The PharmaCare Newsletter is published on the first Wednesday of every month. The PharmaCare Newsletter communicates drug listings, PharmaCare policy, PharmaNet procedures, and other pertinent information for PharmaCare providers and health care partners.
Information in previous newsletters is accurate as of the date it was published. Editions are not retroactively updated when policy, procedures or other information changes. Refer to the most recent mention of a topic for up-to-date information.
PharmaCare providers are required to keep up to date with PharmaCare policies; the PharmaCare Newsletter is critical to staying informed. Subscribe to the PharmaCare Newsletter, read every issue, and share it with staff. It is available online and as a PDF for printing and sharing.
- Subscribe to the PharmaCare Newsletter
- Search past newsletters on the Newsletter search page
BC PharmaCare website
The PharmaCare web pages offer easy-to-read policy summaries and practical guides and procedures for such things as entering claims, Special Authority drug coverage criteria, exceptional coverage for people in the wait period for active MSP enrolment, registration forms for coverage plans, and more.
Definitions for terms in the PharmaCare Policy Manual
A B.C. resident is someone who meets all the following conditions:
- A citizen of Canada or lawfully admitted to Canada for permanent residence
- Makes their home in B.C.
- Physically present in B.C. at least six months in a calendar year. Eligible B.C. residents who are outside B.C. for vacation purposes only, are allowed a total absence of up to seven months a year.
This definition accords with the BC Medical Services Plan’s definition of a B.C. resident.
Active MSP enrolment
A B.C. resident is “actively enrolled in MSP” when they have:
- Completed both steps of MSP enrolment:
- Submitted the Application for Health and Drug Coverage (AHDC) with accepted ID to Health Insurance BC, and
- Presented primary and secondary identification at an ICBC driver licensing office (adults), and
- Reached the end of the MSP wait period, which is two months plus the balance of the month during which they started living in B.C.
MSP coverage is active once MSP enrolment is complete and the resident has completed the wait period, which consists of the balance of the month in which they established residency in B.C., plus two months.
Personal Health Number (PHN)
MSP assigns a 10-digit Personal Heath Number (PHN) to B.C. residents as a unique lifetime identifier for health care. The number is essential to people getting PharmaCare coverage. In rare circumstances, pharmacists can assign PHNs.
- Learn more in Section 3.3 – Patients: Personal Health Numbers
Claim: A request to PharmaCare for payment of the cost of a prescription or pharmacy service such as a dispense
Benefit: A pharmacy service or a product that PharmaCare has reviewed and decided to cover, partially or fully, under a plan, Special Authority, or the Low Cost Alternative or Reference Drug program
Non-benefit: A product that PharmaCare does not cover. Note that non-benefits in the PharmaCare Formulary Search are products that PharmaCare has reviewed and decided not to cover
Common abbreviations in the PharmaCare Policy Manual
- DIN = Drug Identification Number
- LCA = Low Cost Alternative program
- MSP = BC Medical Services Plan
- OTC = Over-the-counter
- PHN = Personal Health Number
- PIN = Product Identification Number
- RDP = Reference Drug Program
- SA = Special Authority (and in some contexts, safer alternative)
 1 – Introduction to BC PharmaCare
1 – Introduction to BC PharmaCare
Section 1 – About BC PharmaCare
What is BC PharmaCare?
BC PharmaCare is British Columbia’s prescription drug program that helps B.C. residents pay for:
- Eligible prescription drugs
- Eligible fees charged by pharmacy providers including
- dispensing fees
- clinical services fees (for renewal or adaptation of a prescription by a pharmacist)
- medication management fees (for specific medication services)
- fees for administering drugs and publicly funded vaccines
- Insulin pumps for people with insulin-dependent diabetes
- Continuous and flash glucose monitors and blood glucose test strips
- Specific insulin pump supplies
- Prosthetic devices (including mastectomy supplies)
- Orthotic devices for children 18 years or younger
- Ostomy supplies
- Certain over-the-counter and prescription smoking cessation products
When a person is considered a B.C. resident for PharmaCare purposes
A person is considered a B.C. resident and eligible for PharmaCare coverage if they meet the residency requirement for the Medical Services Plan (MSP).
>> Learn more about the MSP residency requirement.
Coverage for travel supplies
B.C. residents are eligible for an early “top-up” refill under the Travel Supply Policy once every 6 months (180 days).
>> Learn more in Section 5.4—Travel Supply Policy.
Out-of-province coverage
PharmaCare does not cover eligible benefits for patients who are temporarily out of the province, except:
- Claims meeting requirements under the Section 5.4—Travel Supply Policy
- Claims from patients covered under the Plan W funding arrangement with the First Nations Health Authority (FNHA), under which out-of-province purchases of Plan W benefits may be reimbursed if the client submits a manual claim to PharmaCare
- Certain pre-approved out-of-province expenses when an individual requires treatment not available in B.C.
For example, PharmaCare covers eligible benefit medications and supplies for patients undergoing transplant procedures out of province (provided that PharmaCare receives faxed notification of the surgery from the BC Transplant Society).
In these cases, coverage of out-of-province medications and supplies remains subject to PharmaCare pricing policies and the usual rules of the patient's PharmaCare plan.
Any request for reimbursement for out-of-province purchases should be made in writing and include supporting documents to Health Insurance BC (HIBC).
Who is responsible for BC PharmaCare
The BC Ministry of Health is responsible for the PharmaCare program and sets all policies governing the program.
Health Insurance BC (HIBC) administers the PharmaCare program on behalf of the Ministry. HIBC can answer questions about both the Medical Services Plan and PharmaCare.
How PharmaCare works
PharmaCare offers coverage through 12 plans:
- Fair PharmaCare plan (Plan I)—Income-based coverage for all B.C. residents
- Plan B (Long-term Care) —Permanent residents of licensed long-term care facilities
- Plan C (Income Assistance)—Recipients of B.C. income assistance or in care or a care agreement
- Plan D (Cystic Fibrosis)—Individuals registered with one of four provincial cystic fibrosis clinics
- Plan F (Children in the At Home Program)—Children eligible for benefits through the government's At Home Program
- Plan G (Psychiatric Medications)—For B.C. residents with clinical and financial need
- Plan P (Palliative Care)—Part of the BC Palliative Care Benefits program
- Plan W (First Nations Health Benefits)—For people enrolled with the First Nations Health Authority
- Plan M (Medication Management)—Clinical services provided by pharmacies for B.C. residents
- Plan S (Smoking Cessation)—Smoking cessation products for any B.C. resident
- Plan X (BC Centre for Excellence in HIV/AIDs)—Antiretroviral medications
- Plan Z (Assurance)—100% coverage for all B.C. residents
B.C. residents can be covered under more than one PharmaCare plan. For instance, a B.C. resident with cystic fibrosis may be covered under Fair PharmaCare for most of their eligible prescription and medical supply costs, while also receiving coverage under Plan D for digestive enzymes and nutritional supplements.
Drugs and medical supplies that PharmaCare covers
PharmaCare covers a broad range of prescription drugs. For eligible patients, it also covers certain medical devices and supplies, prosthetics, orthotics, and non-prescription medications.
The drugs PharmaCare covers include eligible medications (as determined by PharmaCare) prescribed by a physician, dentist, midwife, nurse practitioner, naturopath, podiatrist, or optometrist licensed and practicing in B.C.
Smoking cessation products—specific nicotine replacement therapy products—are fully covered for all B.C. residents with active MSP coverage.
>> Check the BC PharmaCare Formulary Search for information on the medications that PharmaCare covers.
More about medical supply coverage
PharmaCare covers the following medical supplies:
- Insulin, needles, syringes, continuous/flash glucose monitors and blood glucose test strips and specific pump supplies—for adults and children with insulin-dependent diabetes
- Insulin pumps and specific pump supplies—for people with insulin-dependent diabetes
- Prosthetic devices (including mastectomy supplies)—for patients of any age
- Orthotic devices—for patients age 18 or younger
- Ostomy supplies—for ostomy patients of any age
>> Learn more about Medical devices and supplies coverage.
Limits on what PharmaCare will cover
To ensure the PharmaCare program is financially sustainable, PharmaCare does not cover all prescription drugs. Instead, it covers drugs based on their effectiveness and cost.
PharmaCare fully covers some drugs (subject to the rules of a patient's PharmaCare plan) but only partially covers other drugs.
For instance, if several versions of a drug contain the same medically active ingredients, PharmaCare may cover only the lower cost versions. If several different drugs can be used to treat the same condition, PharmaCare may cover the more expensive drugs only if the patient has not been helped by the lower cost standard treatment.
Coverage may also be limited by the rules of a patient's PharmaCare plan.
For instance, under the Fair PharmaCare plan, if a patient and their spouse has annual net income above $30,000, they will have to meet a deductible (that is, pay their own drug costs and fees until the deductible is met). Once it is met, PharmaCare covers 70% of their eligible costs and fees for the rest of the year or until the family maximum is met. If the family maximum is met, PharmaCare pays 100% of eligible costs for the rest of the year.
Items PharmaCare does not cover
There are items that are not part of the PharmaCare program. In some cases, PharmaCare has decided not to include an item as a benefit, or coverage may already be provided through another agency. In other cases, the manufacturer has not applied to PharmaCare for coverage of their product.
>> Learn more about what is not covered by PharmaCare.
PharmaCare covers benefits only if they are dispensed by providers enrolled in the PharmaCare program.
How claims are processed
Most claims for prescription drugs and most medical supplies are submitted by a pharmacy or device provider on the provincewide computer network called PharmaNet.
Claims are automatically adjudicated on PharmaNet and the amount PharmaCare pays is deducted from the amount a patient pays when they pick up their product. Patients do not have to submit receipts to PharmaCare.
Any amount PharmaCare is contributing is printed on the pharmacy receipt.
PharmaNet adjudicates both product cost and pharmacy fees.
Pharmacy claims for services to long-term care facilities (capitation fees) and certain medical supplies must be submitted manually.
BC PharmaCare and the Medical Services Plan (MSP)
MSP and PharmaCare are separate programs within the Ministry of Health.
MSP insures medically required services provided by physicians and supplementary health care practitioners, laboratory services and diagnostic procedures. PharmaCare covers eligible prescription drugs, some medical devices and supplies, and pharmacy services.
Other insurers
PharmaCare does not normally cover costs that are fully reimbursed by another payer.
>> For more information, refer to Section 3.4 Patients—Other Payers.

2 – Provider Enrolment in PharmaCare
Section 2.1 – PharmaCare Provider Enrolment
General Policy Description
The Provider Regulation ("The Regulation") under the Pharmaceutical Services Act ("the Act") came into force on December 1, 2014. The Regulation sets out enrolment criteria for pharmacists, facilities, and other places where drugs, devices, substances or related services are provided ("sites").
To submit PharmaCare claims for their patients and to be eligible for any PharmaCare payments, a site (pharmacy or device provider) must apply for enrolment in the PharmaCare program.
Policy Details
PharmaCare makes payments on behalf of beneficiaries for eligible drugs, medical supplies and services, only to providers who:
- Have submitted a HLTH 5432 - Provider Enrolment form (PDF, 585KB) with required documentation, and
- Have subsequently been approved for enrolment in the appropriate class and/or sub-class by the BC Ministry of Health
Provider Regulation definitions
| Beneficiary | A person enrolled in a PharmaCare drug plan |
|---|---|
| Benefit | A drug, device, substance or related service listed on a formulary or Related Services List |
| Claim | A claim for payment that is submitted by a provider to PharmaCare in respect of a benefit provided to a beneficiary |
| Provider | A site (e.g., pharmacy, device provider) that is enrolled in PharmaCare for the purpose of receiving payment |
| Manager |
|
| Class | The two classes of provider established in the Provider Regulation:
|
| Sub-class | Sub-classes within each class, which includes:
|
Who enrols
Any site wishing to enrol as a PharmaCare provider should complete HLTH 5432 - Provider Enrolment form (PDF, 585KB). This allows:
- The site to receive payment for providing PharmaCare benefits to eligible individuals, or
- Eligible individuals to receive payment for PharmaCare benefits provided by their site
A separate Provider Enrolment form must be submitted for each site.
Who does not enrol
- Anyone who needs PharmaNet access only to view patient records (e.g., medical practitioners, emergency departments)
- Inpatient-only hospital pharmacies
- Allergists and allergy laboratories
- Fertility clinics
- Dispensing physicians—unless the College of Physicians and Surgeons of British Columbia (CPSBC) identifies the medical practitioner as a person who should be receiving payment for claims and it would be in the public interest for the medical practitioner to receive payment for claims
Provider obligations—regarding claims
The Act, the Regulation, and the College of Pharmacists of BC (CPBC) bylaws establishes the following obligations for providers:
- The provider must have a designated pharmacy manager, and a valid and subsisting pharmacy licence for the pharmacy at all times.
- All claims submitted to PharmaCare must be submitted in accordance with the provisions of applicable law and College of Pharmacists of BC rules.
- All claims submitted to PharmaCare must contain all information required by PharmaCare.
- All claims submitted to PharmaCare must be true, accurate and complete to the best of the provider’s knowledge.
The provider shall not submit a claim to PharmaCare that the provider knows or reasonably ought to know is false, inaccurate or misleading.
Except where the pharmacy provider is expressly directed or permitted by PharmaCare to submit claims in another manner, the provider must use PharmaNet to submit all claims. The provider shall abide by conditions established by PharmaCare in respect of connection to and use of PharmaNet, including but not limited to PharmaNet professional and software conformance standards.
Providers cannot claim an amount for a benefit that exceeds what the provider would charge to any other person.
>> For further details, refer to the Provider Regulation, Section 17.
Provider obligations—regarding adherence to PharmaCare policy
The provider shall abide by all PharmaCare policies and procedures, provided that they are given reasonable notice of new or revised policies and procedures in advance of implementation through the PharmaCare Newsletter (subscribe to be notified of new editions of the PharmaCare Newsletter).
Provider obligations—regarding inducements
In accordance with the College of Pharmacists of BC’s bylaws, no inducements shall be offered by the provider, or by an agent on behalf of the provider, to any other person to secure prescription orders, or in relation to the provision of a drug, medical supply, or service on the portion of the cost reimbursed by PharmaCare.
“Inducement” means incentives including, but not limited to, cash, points, loyalty points, coupons, discounts, goods, rewards and similar schemes which can be redeemed for a gift or other benefit.
>> Refer to the Pharmaceutical Services Act, Part 5, Section 51(2).
Records and audit
Refer to Section 10—Audit for details.
Ministry change to, or termination of, a provider’s enrolment
Subject to 30 days' notice and an opportunity to be heard, the Minister may:
- Change or cancel a designation made under the Act, or
- Cancel a provider's enrolment
The Minister must give a provider notice of the following:
- An intention to change or cancel a provider's enrolment in a class or sub-class
- An intention to change or add limits and conditions on a provider's enrolment
A provider who receives a notice described above may respond within the period set out in the notice. The response should be in the form of a written submission respecting why the Minister should accept the applicant's application for enrolment, should not change or cancel the provider's enrolment, or should resume payments to the provider, as applicable, and any relevant records or other evidence to support the position of the applicant or provider.
- On receipt of a response from the provider, the Minister must consider the submission, records and evidence provided; may vary, confirm or reverse the decision to deny the applicant's enrolment, change or cancel the provider's enrolment, or take any action the Minister must or may take under the Act, as applicable, and; must give notice to the applicant or provider of the decision or action taken
A notice given by the Minister under the Act is deemed to have been received:
- If sent by registered mail or any other form of delivery, other than personally or electronically, three days after the date the notice was sent,
- If sent electronically, 24 hours after the time the notice was sent
Suspension
The Minister, without giving notice or an opportunity to be heard, may suspend payments owing under Section 45 of the Act by the government to a provider if:
- The provider has engaged in conduct that could be the subject of enforcement action, or has been convicted of a prescribed offence (see below) under an enactment of B.C. or Canada,
- Prescribed circumstances exist (see below), or
- It would be in the public interest to suspend payment
As soon as reasonably practicable after suspending payments, the Minister must give notice of the suspension and an opportunity to be heard.
The Minister must resume payments and pay any amounts owing during the suspension period if the Minister suspends payments:
- On the grounds set out in an enforcement action that is not commenced within three months of the suspension, or
- Following a hearing, the Minister determines that
- the grounds for suspending the payments no longer exist, or
- suspension of payments is unnecessary for any reason or is not required to protect the public interest
If a provider does not respond within 21 days after notice is given or if the Minister does not resume payments after giving an opportunity to be heard:
- The Minister, if applicable, may cancel the enrolment of the provider without giving further notice or an opportunity to be heard, and
- Despite any provision of the Act, or a regulation or an agreement made under it, no further amounts are owing to the provider, and any agreement with the provider is terminated without notice or compensation of any kind
The prescribed offences for suspension of payments are as follows:
- Sections 362, 366, 380, 388, 389, 392, 397, 402, 402.2, 403 and 408 [fraud] of the Criminal Code
- Section 5 [trafficking] of the Controlled Drugs and Substances Act (Canada)
- Section 46 of the Controlled Drugs and Substances Act (Canada), as it relates to a contravention of any provision of said Act
- Part 2 [permitted activities and general obligations of pharmacists] of the Benzodiazepines and Other Targeted Substances Regulations (Canada), SOR/2000-217, or
- Sections 30 to 45 of the Narcotic Control Regulations (Canada), C.R.C., c. 1041
The prescribed circumstances for suspension of payments are as follows:
- If the provider is a corporation and an officer or a director of the provider has been convicted of an offence referred to above
- If a provider becomes ineligible to be enrolled as a provider, except in the circumstances relating to an outstanding audit amount
- In the case of the Opioid Agonist Treatment Provider sub-class: if the pharmacy provider's site is no longer served by pharmacists who are appropriately qualified to dispense medications for opioid agonist treatment (OAT)
- In the case of a device provider sub-class: if the device provider's site is no longer served by a person who is appropriately qualified as described by that section to provide the applicable type of benefit
Provider requests for changes to enrolment
Once you have submitted your application, you are required, as owner of the site, to notify Health Insurance BC (HIBC) of any of the following in accordance with the notification requirement specified in the table below.
Failure to abide by your duties and obligations may result in the delay or suspension of payments.
| Change | Notification requirement |
|---|---|
| Change in provider contact information | Minimum 7 days before change |
| Change of operating/business or corporate name | Minimum 7 days before change |
| Change in owner information | Minimum 7 days before change |
| Change of manager | Minimum 7 days before change |
| Change of location | Minimum 7 days before change |
| Changes to a Power of Attorney | Minimum 7 days before change |
| Cancellation of sub-class | Opioid agonist treatment—30 days before services will end Plan B—No later than the last day of the month before the final full month in which service will be provided Device provider—As soon as reasonably practicable |
| Request to add a sub-class | Recommended notification period: Submit the request at least 21 days in advance of requested effective date to allow for processing. |
| Notice of certain action or event(s)* | Immediately |
| Notice of disposition (sale) or closure | Minimum 30 days before change |
*Actions or events include: order, suspension and/or cancellation of billing privileges, judgment or conviction; suspension or cancellation of pharmacist’s registration and/or pharmacy licence; disciplinary action taken by a governing body or action or proceeding taken by the Canadian Board for Certification of Prosthetists and Orthotists; instances in which an owner of the site has been the director of a corporation that has declared or been petitioned into bankruptcy; and, a requirement to pay an amount to a public insurer, other than BC PharmaCare.
>> Refer to Changing your enrolment information for instructions on how to submit a notice of changes to your enrolment information.
Procedures for Enrolling as a PharmaCare Provider
How to enrol
Information about PharmaCare enrolment and requirements, and associated forms, is on the web page Enrol as a PharmaCare provider. The web page links to the PharmaCare Provider Enrolment Guide and the forms below:
- HLTH 5432 - PharmaCare Provider Enrolment Form (PDF, 585 KB)
- HLTH 5432A - Schedule A (PDF, 513 KB)—Owner Details
- HLTH 5432B - Schedule B (PDF, 512 KB)—Additional Sites
- HLTH 5432C - Schedule C (PDF, 498 KB)—Additional Information
Approved sites become PharmaCare providers and are issued a Site ID (e.g., A01)—a unique identification code issued by HIBC and formerly known as the “Pharmacy/PharmaCare Code/ID.”
Changing your enrolment information
Notify PharmaCare Help Desk of changes to your enrolment information using HLTH 5433 - PharmaCare Provider Change Form (PDF, 746 KB).
>> For notification requirements regarding changes to PharmaNet connections, refer to Section 2.5—Changes to PharmaNet Connections.
Section 2.1 Tools and Resources
- PharmaCare Provider Enrolment Guide (PDF, 260KB)
- HLTH 5432 - PharmaCare Provider Enrolment Form (PDF, 585KB)
- HLTH 5432A - Schedule A (PDF, 513 KB)—Owner Details
- HLTH 5432B - Schedule B (PDF, 512KB)—Additional Sites
- HLTH 5432C - Schedule C (PDF, 498KB)—Additional Information
- HLTH 5433 - PharmaCare Provider Change Form (PDF, 746KB)
- Pharmaceutical Services Act
- Provider Regulation
- Prosthetic and Orthotic Program (device providers only):
- College of Pharmacists of British Columbia Pharmacy Licensure Guide (PDF, 6.5MB)
Section 2.2 – Non-Pharmaceutical Supplier Participation Agreement (Removed)1
1Non-pharmaceutical suppliers now enrol as device providers under the Provider Regulation.
Section 2.3 – What is PharmaNet?
What PharmaNet does
PharmaNet is a secure computer network that links all B.C. community pharmacies and other authorized sites to a central set of databases.
PharmaNet access is available upon request to community and hospital pharmacies, hospital emergency departments, hospitals, designated mental health facilities, medical practices, and non-pharmaceutical and medical device suppliers that are enrolled as device providers with PharmaCare. The levels of permission to access PharmaNet vary.
>> Learn more about the information each type of user can access in Section 9—Privacy.
PharmaNet maintains various types of information, including:
- Patient demographic information
- Patient medication histories
- Drug information
- Drug-to-drug interaction information
- PharmaCare adjudication rules
- Patient clinical information (e.g., allergies, adverse drug reactions) when reported by patients and recorded by pharmacists or authorized physicians and their supervised staff
- Historical patient claims information
The PharmaNet patient profile does not capture information about:
- Drugs dispensed outside community pharmacies, or purchased outside B.C. or over the Internet
- Over-the-counter medications (unless specifically entered)
- Drugs entered under the wrong Personal Health Number (PHN)
- Discontinuations (unless the prescriber or patient has advised the pharmacist)
- Samples provided through physician offices
PharmaNet helps pharmacists to identify and warn patients about potentially harmful medication interactions, unintended duplications, and risks from the misuse of prescription drugs.
The use of PharmaNet is not intended as a substitute for professional judgment. Information on PharmaNet is not exhaustive and cannot be relied upon as complete. The absence of a warning about a drug or drug combination is not an indication that the drug or drug combination is safe, appropriate or effective for any given patient. Health care professionals should confirm information obtained from PharmaNet, and ensure no additional relevant information exists, before making patient care decisions.
When a claim is submitted on PharmaNet, the following is displayed:
- A patient medication history showing medications dispensed in the previous 14 months (or, if preferred, the last 15 dispenses), as well as any over-the-counter medications that may have been recorded
- Drug Use Evaluation (DUE) alerts regarding any potential drug therapy or dispensing problems
>> Learn more in Section 3.14—Drug Use Evaluation.
- All clinical conditions and adverse drug reactions previously recorded on PharmaNet
On request, current patient education drug monographs can also be provided by PharmaNet.
>> Learn more in Section 3.15—Drug Monograph Information.
How PharmaNet adjudicates claims
All prescriptions* dispensed in B.C. community pharmacies must be entered on PharmaNet, whether or not:
- The patient is covered by PharmaCare
- The pharmacy is enrolled as a provider in the PharmaCare program
*HIV/AIDS medications are entered in PharmaNet only when they are dispensed at a community pharmacy. HIV/AIDS medications dispensed at the British Columbia Centre for Excellence are not entered in PharmaNet.
All claims sent by device providers who are connected to PharmaNet must also be submitted through PharmaNet.
At the time an item is dispensed, the provider transmits a claim on PharmaNet and includes the following information:
- Patient (e.g., PHN)
- Prescriber
- Pharmacy or device provider (e.g., the pharmacy Site ID assigned by PharmaCare, security qualifiers)
- Prescription/product information (e.g., product cost, DIN, quantity, days’ supply, drug cost, dispensing fee)
PharmaNet uses the information to adjudicate the claim according to current PharmaCare policies. When adjudicating a claim, PharmaNet:
- Validates the provider’s security authorizations
- Checks whether the patient has a protective word on their PharmaNet record
- Checks the patient’s eligibility for PharmaCare coverage
- does the patient have MSP coverage
- which PharmaCare plan is the patient eligible for
- does the patient have any pharmacy or prescriber restrictions
- Checks the product’s eligibility as a PharmaCare benefit for the patient
- is the product a benefit
- Is it included in the plan(s) for which the patient is eligible
- does the product have any restrictions
- Determines the cost distribution
- how much, if any, of the cost is covered by PharmaCare
- how much, if any, will count toward the deductible
- how much, if any, of a dispensing fee will be paid by PharmaCare
- what portion of the cost is the patient responsible to pay (“co-payment”)
For patients covered under the income-based Fair PharmaCare plan, PharmaNet accumulates any eligible amount of a claim towards the family’s or individual’s annual deductible and family maximum.
Based on the patient’s plan and deductible requirement (if any) and the patient's total expenditures to date, PharmaNet returns the adjudication result to the provider, using standard response/status codes of the Canadian Pharmacists Association (CPhA). These codes indicate how the claim was adjudicated or why it was rejected.
The provider’s local software reports the cost distribution on the prescription receipt.
Note: A provider does not need to know which PharmaCare plan the patient is covered under in order to submit a claim on PharmaNet.
In certain cases, pharmacists and device providers may enter an “intervention” or “exception” code to bypass normal adjudication rules (e.g., for duplicate prescriptions).
>> For the authoritative list of response/status and intervention/exception codes, refer to the Canadian Pharmacists Association (CPhA) Pharmacy Claim Standardt. The Pharmacy Claim Standard may be purchased from Canadian Pharmacists Association at service@pharmacists.ca
Why providers need a local, on-site system
PharmaNet does not replace the need for on-site systems, also known as local systems.
Local systems act as front ends, or the means to access PharmaNet. Local systems may also provide non-PharmaNet features strictly for provider operations.
Providers must use local-system software that a software vendor has had tested and deemed compliant by the Ministry of Health before they can connect to PharmaNet.
>> For more information, refer to Section 2.4—Connecting to PharmaNet.
>> For information on changing your local system software, refer to Section 2.5—Changes to PharmaNet Connections.
Each approved local system software vendor has signed a Service Level Agreement with Health Data Access Services. This agreement sets out problem severity levels and resolution targets. Your software vendor can provide a copy of the agreement by request.
How claims are submitted
Processing a PharmaCare claim involves both your local system and PharmaNet.
Your local system interface determines how a claim is entered.
Learn more about PharmaNet transactions and adjudication in Conformance Standards.
For information on using your local system, contact your software vendor or consult your system documentation.
Section 2.4 – Connecting to PharmaNet
If you need new access to PharmaNet—for the first time ever, or new access after a period without—you need to enrol in PRIME first. This section of the policy manual will soon be updated. For now, see:
- About PRIME and how to enrol
- Private community health practice access to PharmaNet
General Policy Description
All community pharmacies must connect to PharmaNet even if they are not enrolled as PharmaCare providers.
Hospital outpatient pharmacies that wish to submit claims to PharmaCare must connect to PharmaNet.
Device providers have the option of connecting to PharmaNet. Connecting to PharmaNet can simplify device provider claims procedures and improve customer service.
Eligible practitioners within medical practices and facilities such as emergency departments, hospitals and designated mental health facilities may also connect to PharmaNet to obtain dispensing information for their patients. These practitioners do not submit claims to PharmaCare, and cannot view claims information.
Policy Details
All requests for access to PharmaNet must be approved by the Ministry and, for pharmacies and most device providers, the College of Pharmacists of BC (CPBC).
All persons who access PharmaNet must have signed the appropriate agreements and/or undertakings of confidentiality and security before they connect to PharmaNet.
PharmaNet access cannot be granted to sites located outside B.C.
Any online PharmaNet transaction must be processed by the B.C. site (that is, it cannot be processed remotely from another location).
Any personal information obtained from PharmaNet must remain in B.C.
>> For more information on privacy requirements for PharmaNet connections, refer to Section 9—Privacy.
POLICY DETAILS FOR PHARMACIES
Preconditions for PharmaNet connection
All enquiries regarding PharmaNet connection for a new community pharmacy must be directed to the CPBC. This includes new pharmacies that will be part of a gateway system for which PharmaCare does not need to install or connect equipment.
After a pharmacy has made their request through the CPBC, the PharmaCare Help Desk can answer any further questions.
CPBC licensing requirements
Before a pharmacy can connect to PharmaNet, the CPBC must:
- Receive and approve all documentation related to the application for a new pharmacy (including a signed Acknowledgement of Completion of the Confidentiality Procedures, a copy of which will be forwarded to PharmaCare), and
- Receive payment of the licence fee.
>> Refer to the full list of required documents in the CPBC Pharmacy Licensure Guide (PDF, 6.5MB).
The CPBC notifies PharmaCare of its preliminary approval of each new pharmacy’s licence application. The PharmaCare Help Desk can then initiate the PharmaNet connection process.
Pharmacy point-of-sale (POS) software requirements
Pharmacies must use approved, compliance-tested software to connect to PharmaNet.
Conformance evaluations are performed by HIBC and the staff of Data Access, Research and Stewardship (Health Sector IM/IT Division, Ministry of Health) plus a member of a regulatory body where appropriate. The evaluation considers all aspects of PharmaNet functionality available on the local software, whether or not all functions are/will be used by the pharmacy to confirm that:
- The local software complies with requirements
- Local system functions and processes provide accurate results
- Pharmacies must have selected an approved software vendor before they submit their request for connection; the vendor’s name must be provided as part of the application.
>> Refer to the list of approved software vendors.
Confidentiality undertaking requirements
Before PharmaNet connection can proceed, the Pharmacare Help Desk must receive a copy of the pharmacy-signed confidentiality document (Acknowledgement of Completion of the Confidentiality Procedures) from the CPBC. A signed confidentiality document cannot be accepted directly from the pharmacy.
Enrolment as a PharmaCare provider is required for billing to PharmaCare.
Connection requirements
All software applications used to access PharmaNet, including pharmacy PharmaNet applications (e.g., Kroll, WinRx, Propel), private community health practice PharmaNet applications (e.g., Medinet, CareConnect), and health authority PharmaNet applications (e.g., Cerner, Meditech) connect to PharmaNet through the PharmaNet API. PharmaNet API is the approved, secure connector for PharmaNet that provides appropriate security for connections to PharmaNet over public internet infrastructure.
PharmaNet API connections cannot be completed independently. The Ministry of Health and software vendors together determine a date and time to set up the connection.
To connect to PharmaNet through the internet, pharmacies and other PharmaNet access sites must use only software applications that have been conformance tested and approved by the Ministry of Health. Refer to Conformance Standards for further details.
All PharmaNet access sites are responsible for obtaining internet connection services from an ISP using industry-standard privacy and security best practices for providing reliable internet services. They are also responsible for having their local system software vendor install and test the PharmaNet connection software before they access PharmaNet.
Connection and activation time frames
Your system vendor can provide an estimate of the time it will take them to set up and test your local system’s connection.
Upon approval of all documentation and payment of licence fees, the CPBC will notify PharmaCare. PharmaCare will register the pharmacy in the PharmaNet security system, ready for activation. This process may take up to 10 business days.
Activating the connection to PharmaNet
Connection to PharmaNet will be activated once the CPBC notifies the PharmaCare Help Desk that the Acknowledgement of Completion of the Confidentiality Procedures form has been submitted.
POLICY DETAILS FOR OTHERS:
Device providers
PharmaNet allows device providers who have enrolled with PharmaCare to submit online claims but does not allow them to access a patient’s full medication history.
The benefits of connecting to PharmaNet include:
- Claims on PharmaNet adjudicate in real time, providing immediate information on the portion of a claim to be paid by the patient and the portion (if any) covered by PharmaCare;
- Not having to submit manual claim forms; and
- Eliminating the two- to three-week turnaround time required for processing manual claims and issuing payment for them.
Connection requirements and time frames
Device providers must have enrolled with PharmaCare before they can request access to PharmaNet and submit claims. For enrolment details, refer to “PharmaCare Provider Enrolment” on the Information for device providers webpage.
For general information on the connection requirements and time frames, refer to the process for pharmacies above. For more detailed information, refer to the PharmaCare Prosthetic and Orthotic Policy Manual.
To request access to PharmaNet, contact the PharmaCare Help Desk.
Insulin pump manufacturers/distributors
Insulin pump manufacturers/distributors (formerly “Medical Device Distributors”) may sell their products either directly to the patient or to an authorized vendor such as a pharmacy or medical supply store.
To participate in PharmaCare, insulin pump manufacturers/distributors must enroll with PharmaCare and may be granted limited access to PharmaNet.
>> Learn more about registering for access to PharmaNet in Medical device distributors access to PharmaNet.
Out-of-province sites
Out-of-Province (OOP) sites are not connected to PharmaNet.
However, PharmaCare may allow OOP sites to participate in PharmaCare. These sites are closer than the nearest provider located in B.C., serving B.C. residents in border communities.
OOP sites must submit manual claims to PharmaCare in the same way as in province device providers that are not connected to PharmaNet.
>> Refer to Section 4.1—Claims by Offline and Out-of-Province Suppliers and Section 7.10—Submitting Claims, Prosthetic and Orthotic Policy Manual.
Community health practices
Community Health Practice Access to PharmaNet (ComPAP) allows authorized health care professionals to request and receive up-to-date records of medications dispensed to a patient, in a timely and secure manner, at each registered community health practice site in B.C.
Access to PharmaNet aims to enhance patient care by providing community health practice staff with complete, accurate and comprehensive patient and drug information.
This service is available for health practitioners in community health practices and supervised persons acting on their behalf.
Practitioners may register to access PharmaNet from one or more sites at which they practice. They may access PharmaNet only from within the sites for which they have registered.
Practitioners who wish to access PharmaNet from health authority facilities, refer to PRIME and Health authority facility access to PharmaNet.
>> Learn more about Community health practice access to PharmaNet.
Emergency departments
This service permits authorized individuals in hospital emergency departments, and diagnostic and treatment centres, to access patient medication profiles to assist in determination of patient therapy, in a timely and secure manner.
>> Learn more at Health authority facility access to PharmaNet.
Hospitals
Hospital Access to PharmaNet (HAP) allows authorized physicians and pharmacists to request and receive up-to-date records of medications dispensed to a patient, in a timely and secure manner, at each registered hospital or designated mental health facility (DMHF).
This service is available for physicians and pharmacists in a hospital or DMHF and authorized persons acting on the physician's behalf.
>> Learn more about Health authority facility access to PharmaNet.
Section 2.5 – Connecting to PharmaNet
General Policy Description
All PharmaNet access sites must be registered in PRIME, and the registration information must be kept current to maintain PharmaNet access.
Policy Details
Business changes
Owners of a PharmaCare-enrolled provider must notify PharmaCare of business changes (such as changes to ownership and management) using the PharmaCare Provider Change Form (HLTH 5433) (PDF, 746KB).
Failure to report changes may result in delay or suspension of payments.
PharmaNet access sites
Under the Organization Agreement for PharmaNet Access signed when a site is registered in PRIME, an organization must immediately notify the Province:
- If a PharmaNet access site or any portion of a site is leased or transferred to another person
- If the organization ceases to operate a site
- If the organization acquires a premises that already has PharmaNet access, whether or not the organization intends to provide site access from that location
- Of any change to the information in site registration details, including changes to the organization that owns/operates the site, site name, site status, location, operating hours, PharmaNet software vendor, name/contact for signing authority, PharmaNet administrator, privacy officer, or technical support contact of a change of control of the organization
If you make a change to a PharmaNet connection without reporting it in time to the PharmaNet Help Desk and/or PRIME, you may lose connection to PharmaNet.
Reporting changes
All changes must be reported and approved by the Ministry beforehand. The appropriate contact varies depending on the change and some changes must be reported to multiple contacts.
Changes to report to the PharmaCare Help Desk
Visit PharmaCare Help Desk for contact details.
- PharmaNet software version update with same vendor (2 business days before the update)
- Closing a site (30 days before the closure)
Changes to report to PRIME Support
Email PRIMEsupport@gov.bc.ca to report the following changes.
- Operating/business, corporate name, or site name change (at least 30 days before change)
- Change from one vendor's software to another vendor's (contact PRIME to discuss at least 3 months ahead of the proposed change date)
- Relocating to new premises (address change) – (timeline depends on the situation. Contact PRIME to discuss well in advance of the change)
- Closing a site (30 days before the closure)
- Disposition of a site (30 days before disposition; requires a new site registration by the new owner in PRIME)
Changes to report by submitting HLTH 5433 – PharmaCare Provider Change form
These changes are reported using HLTH 5433 – PharmaCare Provider Change (PDF, 746KB) form in advance of the change.
- Provider contact information (7 days before change)
- Operating/business or corporate name (7 days before change)
- Owner information (7 days before change)
- Manager (7 days before change)
- Location (7 days before change)
- Power of attorney (7 days before change)
- Cancellation of sub-class
- Opioid Agonist Treatment: 30 days before services end
Plan B: Before the last day of the month before the final full month of service
Device provider: As soon as possible
- Opioid Agonist Treatment: 30 days before services end
- Request to add sub-class (7 days before effective date)
- Notice of certain actions or events - immediately after the event
- Order, suspension and/or cancellation of billing privileges judgment or conviction
- Suspension or cancellation of pharmacist’s registration and/or pharmacy licence
- Disciplinary action taken by a governing body or action or proceeding taken by the Canadian Board for Certification of Prosthetists and Orthotists
- Instances in which an owner of the site has been the director of a corporation that has declared or been petitioned into bankruptcy
- A requirement to pay an amount to a public insurer, other than BC PharmaCare
- Disposition or closure
- Disposition: 30 days
Closure: 30 days
- Disposition: 30 days
Section 2.6 – Dealing with PharmaNet Network Outages
PharmaNet outages
If PharmaCare knows that PharmaNet is going to be unavailable—for example, to make emergency changes—the PharmaCare Help Desk attempts to notify affected users, either by telephone (if the number of users affected is limited) or through a fan-out message (if all users will be affected).
Emergency change windows are communicated using a fan-out message, should time permit.
Regular change window
An eight-hour maintenance interval is scheduled every Thursday morning, from 12:01 am to 8 am. This change window is required for routine and scheduled maintenance on PharmaNet.
Any alteration to this regular change window is communicated in the PharmaCare Newsletter.
Business options when PharmaNet is unavailable
Occasionally, PharmaNet is unavailable. Network disruptions can arise from power cuts, problems with regional or core routers, phone line problems, an unexpected spike in transaction volumes, or failures/errors in computer hardware or software.
Normally, the first sign of a network disruption is a message from the local system after it attempts to communicate with PharmaNet but fails. The system then notifies you that the network is unavailable (with error messages such as “timeout” or “not connected to host”).
Pharmacies have two options when PharmaNet is unavailable (“down”):
- Dispense prescriptions offline—Enter prescriptions into your local system until PharmaNet is available. This option allows you to operate almost normally but you will not have access to Drug Use Evaluation (DUE) or adjudication results until PharmaNet becomes available.
- When PharmaNet is back online, your local system sends the accumulated transactions as a batch to PharmaNet for DUE checking and adjudication.
- As soon as convenient, review the DUE results and take action as appropriate.
Refer to Dispensing offline below for details.
- As soon as convenient, review the DUE results and take action as appropriate.
- Stop dispensing prescriptions until PharmaNet is back online. This disrupts pharmacy business but may be necessary if, for example, your local system is also inoperable.
Device providers can submit claims for adjudication once PharmaNet is operational again.
Dispensing offline
Processing prescriptions offline calls on professional judgment in treating each patient. Pharmacists must also decide how to deal with payments.
Since there are no pre-set deductibles for Fair PharmaCare, it may be difficult for pharmacies to anticipate an individual’s level of PharmaCare coverage.
Offline payment options
When operating offline, a pharmacy can flag a claim as “pay provider” or “pay cardholder.” When the batched claims are transmitted to PharmaNet and claims are adjudicated, the pharmacy (provider) or patient (cardholder) is reimbursed appropriately.
| Pay cardholder | Collect payment from the patient. PharmaCare will automatically reimburse the patient appropriately. |
| Pay provider | Do not collect payment from the patient. PharmaCare will automatically reimburse the pharmacy. |
You may choose to dispense the full prescription or an emergency supply that will tide the patient over until PharmaNet is operational again.
Processing prescriptions offline when the PHN is unknown
Pharmacy software restricts your ability to process a prescription without the patient PHN. When PharmaNet is unavailable, a PHN search is not possible, and a PHN cannot be assigned.
To process a prescription claim offline when PHN is unknown:
- Submit the prescription claim as usual, entering 0009999999998 as the PHN.
Once PharmaNet is online, the local system submits the batched transaction to PharmaNet. PharmaNet will reject it. - When PharmaNet rejects the batched claim, search for, or assign, a valid PHN as if processing the prescription normally.
- Re-submit the claim on PharmaNet.
Note: PharmaNet will return the patient medication history and Drug Use Evaluation (DUE) results. - As soon as convenient, review the medication history and DUE results, and take appropriate follow-up action as required.
Section 2.7 – Fan-Out Messages
The Ministry of Health sometimes sends urgent messages through PharmaNet to community pharmacies in B.C. Such “fan-out” messages are transmitted to all pharmacies or to specific groups of pharmacies, e.g., by geographical area.
Fan-out messages may be sent in case of a:
- Lost prescription pad (regular or duplicate)
- Stolen prescription pad (regular or duplicate)
- Unscheduled PharmaNet outages (i.e., outside of a scheduled change)
Fan-out messages are automatically received by pharmacy PharmaNet software and displayed on the screen for immediate printing. The message should be printed out and made available to all pharmacy staff to reference.
Reporting a lost or stolen prescription pad
Only registrants of the College of Physicians and Surgeons of BC, the BC College of Nurses and Midwives, the College of Pharmacists of BC, or a dentist licensed by the BC College of Oral Health Professionals can report a lost or stolen prescription pad (regular or duplicate).
To report a lost or stolen prescription pad:
- Call PharmaNet Data Quality Services Team at 1-844-660-3200. The phone line is answered Monday to Friday, 8 a.m. – 4 p.m. Outside of these hours, leave a voice message.
- Provide the prescriber name and location, Prescriber ID Reference Code and licence number; relevant folio number(s); contact information; and additional information as required.
- Within one business day, the PharmaNet Data Quality Services Team will issue a fan-out to pharmacies in the prescriber’s geographical area.
Other urgent information
PharmaNet Data Quality Services Team does not issue a fan-out about incidents other than those outlined above. For example, fan-outs are not issued for prescription forgeries, pharmacy robberies, persons impersonating health care providers or insurers, multi-doctoring.

3 – Claims Submission
Section 3.1 – Which Transactions to Submit in PharmaNet
General Policy Description
Some transactions (claims and/or information) must be submitted in PharmaNet; some transactions may be submitted in PharmaNet at the provider’s discretion. Some transactions must not be submitted in PharmaNet.
Manually submitted claims are entered in PharmaNet by Health Insurance BC (HIBC).
Overview
Most transactions for pharmacy products or services delivered or dispensed directly to a client (e.g., prescription drugs and clinical services) must be submitted in PharmaNet.
A pharmacy’s office-use medication (O-Med) transactions must also be submitted in PharmaNet.
Transactions for pharmacy products or services that are not delivered or dispensed directly to a client (e.g., stock transfers, returns) should be recorded only on the pharmacy’s local system.
Transactions that must be submitted in PharmaNet include:
- All claims for prescription items, including compounded prescriptions
- Claims for blood glucose test strips
- Claims for insulin pump supplies
- Claims for nicotine replacement therapy (NRT) products covered under the Smoking Cessation Program
- Claims for clinical services, including pharmacist adaptation and renewal of prescriptions, administration of publicly funded vaccines, and medication review services
- Sales of O-Meds to clinics/practitioners
For details, refer to Transactions that must be submitted in PharmaNet
Transactions that should not be submitted in PharmaNet include:
- Stock transfers from one pharmacy to another, including emergency supplies of narcotics and controlled drugs
- Sales of inventory to other pharmacies
- Transfers of inventory from a pharmacy to a long-term care facility
- Drug returns to wholesalers
For details, refer to Transactions that should not be submitted in PharmaNet
Transactions that may be submitted in PharmaNet OR submitted manually include:
- Claims for prostheses and orthoses
- Claims for ostomy supplies
- Claims for insulin pumps
For details, refer to Transactions that may be submitted in PharmaNet OR submitted manually
Transactions that must be submitted manually include:
- Claims for patients using out-of-province (OOP) sites that are enrolled in PharmaCare (these sites cannot connect to PharmaNet)
For details, refer to Section 4—Offline (Manual) Claims
Policy Details
TRANSACTIONS THAT MUST BE SUBMITTED IN PHARMANET
Claims for prescription medications
All prescription medications dispensed in B.C. community pharmacies must be entered in PharmaNet, whether or not the product or client is covered by PharmaCare.
Recording all dispenses in PharmaNet ensures that:
- A client’s current medication history is available to authorized healthcare practitioners
- Authorized healthcare practitioners can accurately identify potential drug-to-drug interactions and check for previous adverse effects from specific drugs
Prescription medications include both compounded prescriptions and medications sold to clinics for office use (refer to Office-Use Medications).
Claims for NRT products covered under the Smoking Cessation Program
To obtain PharmaCare coverage for nicotine replacement therapies for a patient, pharmacies must enter a claim in PharmaNet at the time of purchase using the pharmacist’s Pract ID and the appropriate PIN.
For details, refer to Section 5.20—Smoking Cessation Program Policy.
Claims for clinical services
Pharmacy claims for clinical services must be entered in PharmaNet with the appropriate intervention codes and/or PINs.
For details, refer to:
- Section 8.4—Clinical Services Fees
- Section 8.9—Medication Review Services
- Section 8.10—Pharmacist Administration of Drugs and Vaccines
Office-use medications (O-Meds)
All O-Meds sold to clinics/practitioners by a community or hospital outpatient pharmacy must be transmitted in PharmaNet using the pharmacy’s unique O-Med PHN and the corresponding keyword designated for that pharmacy.
O-Med PHNs
A pharmacy that has not been assigned a unique O-Med PHN may obtain one by phoning the PharmaCare Help Desk.
Keywords associated with the O-Med PHN can be changed using the Patient Keyword Maintenance (TCP) transaction.
Prescriptions for a practitioner’s personal use must be dispensed using the practitioner’s own PHN, not the pharmacy’s O-Med PHN.
O-Med medication histories
Any questions regarding O-Med medication histories (or any other medication history) in PharmaNet should be directed to the College of Pharmacists of BC (CPBC).
A pharmacy may use the last-15-prescriptions option (TRR) to review the PharmaNet medication history for its O-Med PHN.
Dispensing O-Meds
When transmitting claims to PharmaNet for O-Meds, pharmacists should use:
- O-Med PHN and keyword
- Date of dispense
- Intervention Code UA (to eliminate the "duplicate" message if dispensing the same DIN on the same day)
PharmaNet returns an error message for O-Med transactions because claims for O-Meds are not adjudicated by PharmaCare.
PharmaNet does return Drug Use Evaluation messages for O-Med transactions.
Reversing O-Med transactions
To reverse an O-Med transaction, use the O-Med PHN, the keyword, and the Intervention Code RE.
For more information, refer to Section 3.16—Claims Reversals
Claims for insulin pump supplies
Only providers may submit claims for insulin pump supplies (i.e., infusion sets/kits and reservoirs/cartridges). Patients cannot submit manual claims for insulin pump supplies.
For coverage details, refer to Section 5.18—Insulin Pump Supplies
For procedures, refer to the PharmaCare Claims For Insulin Pump Vendors Quick Reference Guide (PDF, 1.2MB)
TRANSACTIONS THAT SHOULD NOT BE SUBMITTED IN PHARMANET
Stock transfers from pharmacies
Stock transfers include the sale of drug inventories to other pharmacies, including emergency supplies of narcotics and controlled drugs, the transfer or sale of drugs to long-term care facilities, and returns of stock to drug wholesalers.
Stock transfer functions are unique to each pharmacy’s local software; direct any questions to your pharmacy software vendor rather than to the PharmaCare Help Desk.
Stock transfer transactions must not be transmitted in PharmaNet. The record of such transactions must be captured on the local pharmacy system only.
A pharmacy must not use its O-Med PHN or assign a PHN through PharmaNet for the purpose of stock transfers.
Sale of inventory to other pharmacies
The sale of drug inventory between pharmacies should only be recorded in the local system and should not be transmitted in PharmaNet. The local system software must record the sale of emergency quantities of all drug inventories.
Transfer of inventory from a pharmacy to a long-term care facility
The transfer of inventory to a long-term care facility for future use by the facility should only be recorded in the local system. The stock transfer must not be transmitted in PharmaNet.
The facility’s pharmacist should transmit the patient and prescription information in PharmaNet only when medication is dispensed to a patient in a long-term care facility.
Pharmacy stock returns to wholesaler
The return of drug inventory to the wholesaler should only be recorded on the local system and should not be transmitted in PharmaNet.
TRANSACTIONS THAT MAY BE SUBMITTED IN PHARMANET OR SUBMITTED MANUALLY
Claims for prostheses and orthoses
Claims for prostheses and orthoses may be made by device providers such as prosthetists, orthotists, ocularists, anaplastologists, mastectomy fitters and/or their companies or businesses if they are enrolled in the appropriate sub-class.
For more information, refer to the Prosthetic and Orthotic Policy Manual.
Claims for ostomy supplies
Pharmacy and device providers enrolled in the appropriate sub-class may submit claims for ostomy supplies.
For details, refer to Section 5.21—Ostomy Supplies.
Claims for insulin pumps
Device providers enrolled in the appropriate sub-class may submit claims for insulin pumps for eligible patients.
For details, refer to Section 5.17—Insulin Pumps.
ADDITIONAL INFORMATION THAT CAN BE ADDED TO A PATIENT'S PHARMANET MEDICATION HISTORY
Over-the-counter (OTC) and no-public-access (NPA) medications
At the discretion of a pharmacist or medical practitioner, over-the-counter (OTC) and no-public-access (NPA) medications may be added to a patient’s medication history.
Pharmacists must use their CPBC licence numbers in the Pract ID field and “P1” in the Pract ID Ref field.
As with prescription drugs, entering OTC and NPA medications on a patient’s medication history allows monitoring of medications and use of the Drug Use Evaluation (DUE) function.
Procedures
Procedures for all users
Submitting transactions in PharmaNet
If you have access to PharmaNet, enter the transaction in your local system and then submit it in PharmaNet.
Submitting a manual claim for a product or service
If you are enrolled as a PharmaCare provider but do not have access to PharmaNet:
- Submit a manual claim for the product or service, or
- Provide the patient with sufficient information and documentation (i.e., receipts) so that they may submit a manual claim to PharmaCare
For details, refer to Section 4—Offline (Manual) Claims.
Procedures for pharmacists
Entering OTC and NPA medications in PharmaNet
In the Pract ID field, enter your pharmacy’s CPBC licence number.
- In the Pract ID Ref field, enter P1.
Section 3.2 – Patients - Identification
General Policy Description
New patients must be properly identified before a claim can be submitted.
Policy Details
Before creating a patient record, the pharmacist must take all reasonable steps to positively identify the patient, or the patient’s personal representative, in compliance with College of Pharmacists of BC guidelines and, for the management of patient protective words, the provincial government’s Office of the Chief Information Officer Evidence of Identity Standard (PDF, 1.4MB).
Refer to Section 9.1—Positive Identification of Patients for full information on requirements.
Before a pharmacist can fill a prescription for a new patient of the pharmacy, the pharmacist must enter the patient’s PHN and create a patient record on the local pharmacy system.
Procedures for Pharmacists
Processing a prescription for a new patient
- If the patient has a BC Services Card, or knows their PHN, jump to step 4.
- Determine whether the patient has a Confirmation of Application for Medical Benefits form or other confirmation of assistance document from the Ministry of Social Development and Poverty Reduction.
- If the patient has the necessary documentation, go to Processing New Plan C Patients in Section 7.4—Income Assistance (Plan C). Otherwise, go to step 4.
- Search PharmaNet for the patient’s PHN as described in Section 3.3—Searching for a PHN.
- If the search is unsuccessful but the patient states they do have a PHN, or if you cannot distinguish the correct PHN from multiple matches, go to Section 3.3—Searching for a PHN.
- If the search verifies that the patient does not have a PHN, assign a PHN according to the steps in Section 3.3—Assigning a PHN.
- Complete the transaction as a normal claim.
Section 3.3 – Patients - Personal Health Numbers
General Policy Description
In British Columbia, each client needs a Personal Health Number (PHN) in order to access medical care, including prescription drugs.
Policy Details
PHN requirements for processing prescriptions
To process a prescription in PharmaNet, the client's PHN is required.
Pharmacist-assigned PHNs
Every reasonable effort must be taken to obtain a client’s PHN. This includes asking the client (or a relative), searching local files, performing a name search on PharmaNet, and, if necessary, calling the prescribing physician and/or the PharmaCare Help Desk (refer to How to Search for a PHN).
If a pharmacist is certain that a client does not have a PHN, they can assign one through PharmaNet.
PHNs must be assigned only for the following types of clients:
- Non-residents of B.C. who have not lived in or used a B.C. health service (e.g., lab work, hospital visit, prescription fill) since 1995 (which is as far back as PharmaNet can search)
- B.C. residents who do not have BC Medical Services Plan (MSP) coverage
- Newborns (in rare cases)
Be cautious in assigning PHNs. Perform a thorough search in PharmaNet before concluding that a PHN does not exist.
- The creation of duplicate PHNs is a serious data integrity problem that may deprive clients of benefits and endanger their health (refer to Multiple PHNs Assigned to One Patient).
- If PharmaNet is offline and preventing you from performing a search or assigning a PHN, refer to Section 2.6—Dealing with PharmaNet Network Outages, Dispensing Offline.
PHNs versus MSP coverage and PharmaCare eligibility
To receive medical care (including prescription drugs) in B.C., every client must have a PHN whether or not they are a B.C. resident.
However, having a PHN does not mean the client is eligible for MSP or PharmaCare coverage. PHNs created by pharmacies do not entitle the client to B.C. medical coverage or PharmaCare coverage and are treated in the same way as PHNs for out-of-province clients. Claims for prescriptions filled under these PHNs adjudicate to $0.00.
Any PHN assigned at the pharmacy becomes the client’s permanent health identifier and is used by MSP and PharmaCare if the patient obtains assistance in the future.
Prescriptions for a client without MSP coverage adjudicate to $0.00. That is, the patient’s expenditures do not count toward the annual Fair PharmaCare deductible, even if they register later for Fair PharmaCare.
Prescriptions for a client who has MSP coverage but who has not registered for Fair PharmaCare will also likely adjudicate to $0.00. Clients who have not registered for Fair PharmaCare have a default deductible of $10,000. However, if the client registers for Fair PharmaCare before the end of the calendar year and submits a consent form within the requested timelines, the client’s eligible expenditures for the rest of the year will count toward their Fair PharmaCare deductible.
For information about the adjudication of claims for individuals who are enrolled in MSP but who are not registered for Fair PharmaCare, refer to the coverage start date information under Registration, in Section 7.2—Fair PharmaCare.
Cancellation of residency status
If a client’s MSP coverage is cancelled because they have taken up permanent residence outside of B.C., they are ineligible for PharmaCare assistance, even if they have a PHN and still possess a BC Services Card. In these cases, the patient’s PharmaCare claims adjudicate to $0.00 and they will have to pay in full.
Non-residents
A client does not have to be a B.C. resident to receive a PHN.
Visitors from another province or country who need to fill a prescription in B.C. must be assigned a PHN before the prescription can be dispensed.
Before assigning a PHN for a non-resident, perform a name search to ensure that the client has not been assigned a PHN at another pharmacy or another healthcare point of service during an earlier visit to B.C., or during a previous period of residency in the province.
Veterinary prescriptions
PHNs must not be assigned to animals.
When dispensing a prescription for an animal, use the animal owner's PHN and the veterinarian’s veterinary association ID number. The dispense will not affect the animal owner’s patient record or Drug Use Evaluation (DUE) results or contribute to their Fair PharmaCare deductible or co-pay.
Non-patient supplies
PHNs must not be assigned for stock transfers, office-use supplies or emergency supplies. For more information, refer to Which Transactions to Submit in PharmaNet.
PHNs must not be assigned to pharmacies, practitioners’ offices, clinics or facilities.
Procedures for Pharmacists
No prescription can be processed in PharmaNet unless the client has a PHN.
Any client who is a resident of B.C. and has enrolled in MSP has a PHN. A PHN may also exist for clients visiting from outside the province, either because they used a B.C. health service (e.g., lab work, hospital visit, prescription fill) or lived in B.C. at some time since 1995 (which is as far back as PharmaNet can search).
You must make every reasonable effort to obtain a client’s PHN. This includes asking the client (or client’s relatives), searching local files and/or performing a name search in PharmaNet. Refer to the procedure below or to the Quick Guide. As a last resort, call the prescriber and/or the PharmaCare Help Desk.
If the client or their authorized representative does not have the client’s BC Services Card or know their PHN, you will need to perform a name search.
- Confirm the client’s or representative’s identity in accordance with the requirements set forth in Section 9.1—Positive Identification of Patients.
- Access PharmaNet and perform a Patient Name search (TPN transaction) using
- Full surname
- Full first name
- First name initial (see below)
- Date of birth
- Gender
- If only one PHN is returned, verify it by cross-checking the client’s full name and address.
- If the name search does not find a PHN, perform an advanced name search:
- Make sure the first and last name on the prescription matches the name on the identification
- Ask if the client is married or divorced and/or has changed their last name
- Ask if the client has a hyphenated name (e.g., with spouse’s name)
- Check for embedded spaces in the last name (e.g., van der Ham vs. Vanderham)
- Search under the client’s first name and/or second-name initial with a surname in the Last Name and Given Name fields (e.g. Michelle Georgina Bertelli can be searched under M. Bertelli or G. Bertelli)
- Search using the client’s full middle name (e.g., Georgina Bertelli)
- Check whether the client uses a nickname, which might begin with a different letter (e.g. Robert may be entered as Bob or Bobby)
- Try variant spellings of the given name (e.g. Chris may be Christopher, Christophe or Krystof)
- Try closely related names (e.g. Mac vs. Mc)
- Switch the client’s first name or second name with their surname; many cultures record the family name first and the given name second
- For people with only one legal name, enter that name in both the Last Name and Given Name fields. If that does not work, enter the client’s legal name in the Last Name field and a title (e.g., Mr., Ms.) in the Given Name field
- Confirm the birthdate. The system returns results only for the year given. The year must be exact
- If the patient is a newborn, a PHN may exist, but under a different surname. Ask for any other name the newborn may be registered under. For newborns, enter "Baby Girl” or “Baby Boy” in the First Name field
If you still cannot find the PHN and the client is a B.C. resident, contact the prescriber. If the prescriber does not have sufficient information, call the PharmaCare Help Desk.
If PharmaCare Help Desk cannot find a PHN, you can assign one. Refer to Assigning a PHN.
If you find multiple PHNs for the same client
Follow the procedure in Multiple PHNs Assigned to One Patient.
If the client information appears to be wrong
If the search returns information that does not match the information provided by the client, check that you have the correct PHN.
If the mailing address or phone number is incorrect or outdated, update them using the Patient Address Update (TPA) function.
If any information other than the above needs to be corrected, refer the patient to the:
- Medical Services Plan of British Columbia
- B.C. Vital Statistics Agency
- For non-B.C. residents, contact the PharmaCare Help Desk
Print off the one-page guide, How to Searching for a PHNs in PharmaNet.
Only pharmacists and the Ministry of Social Development and Poverty Reduction can assign PHNs. The PharmaCare Help Desk cannot assign a PHN.
To reduce the creation of multiple records, the pharmacy or acute healthcare point of service must accurately identify the patient before assigning a new PHN.
>> Refer to Section 3.2—Patients – Identification.
Before assigning a new PHN, pharmacists must perform a thorough PHN search.
If a client cannot provide a BC Services Card, and you are sure a PHN does not already exist, a PHN may be assigned.
If the client is incarcerated (in a federal or provincial facility) please do not create a duplicate PHN. Use the PHN that the demographics (name, gender, DOB) match, even if the address is different.
Before assigning a PHN, the client must have been positively identified. See Section 9.1—Positive Identification of Patients.
Assign the new PHN using the full name that appears on the client’s identification documents. Do not use initials or nicknames. The use of first initials instead of a full name is a major cause of duplicate PHNs.
When a client has only one legal name, enter the one name twice, in both the Last Name and Given Name fields.
The address recorded must be the client’s mailing address – no PO boxes.
PATIENT NAME FOR NEWBORNS
PHNs are typically assigned to newborns at birth. However, if a baby has not been assigned a PHN, a pharmacist may assign one that conforms to the rules below.
Newborn surname
If known, the baby’s legal surname must be entered.
If not known, use the mother’s legal surname.
Newborn given name
If known, the baby’s legal given name must be entered.
If not known, the baby’s legal given name must be entered as:
- For single births: BABY BOY or BABY GIRL
- For multiple births: The appended letter must indicate the sequence of birth. For example, for triplets: BABY BOY A, BABY GIRL B, BABY BOY C
The baby’s legal given name will appear in PharmaNet once HIBC has received the request for coverage.
Address
The patient address should be their mailing address.
For out-of-province or out-of-country patients, record their permanent home mailing address and not their temporary visiting address in B.C.
Assigning a new PHN
- Collect positive patient identification.
For a list of acceptable identification, refer to Section 9.1—Positive Identification of Patients. - Assign a new PHN in PharmaNet..
- The minimum information required to generate a PHN is:
- surname
- full first name (not a nickname) OR a first initial only when the person does not have or know the first name. Initials should be used only as a last resort
- mailing address
- municipality, city or town
- province
- country
- postal code
- date of birth
- gender
- The minimum information required to generate a PHN is:
- Assign the new PHN to the local system and process the prescription as usual.
If the client is a B.C. resident who has never been enrolled in MSP, refer them to MSP to enrol (otherwise, PharmaNet will list the client as a non-resident).
MULTIPLE PHNs ASSIGNED TO ONE CLIENT
On occasion, more than one PHN is assigned to the same person. Records of the PHNs previously assigned to a client are displayed, so there may be multiple matches with the same PHN.
If you suspect a duplicate, contact the PharmaCare Help Desk.
Multiple PHNs for the same person may, for example:
- Cause inaccurate information about deductibles, as the patient’s accumulated expenditures are divided between the two or more PHNs
- Create a split in the patient record, affecting DUE checks.
- Deprive patients of government and private insurance benefits,
- Compromise their health care in an acute or long term-care hospital, emergency room, doctor’s office or walk-in clinic (e.g. the drug interaction check fails to catch potentially harmful combinations as the medication history is incomplete)
IF YOU FIND MULTIPLE PHNs FOR A SINGLE CLIENT
- Ensure you have searched with the correct full name and address.
- Confirm the name and address with the client and find the exact match.
- If there is no exact match, verify client details such as address, telephone number and date of birth.
- If there is more than one exact match under different PHNs and you are satisfied that they are the same person, call the PharmaCare Help Desk for instructions on which one to use.
Note: The PharmaCare Help Desk applies complex criteria to determine which PHN to retain when two records are merged. HIBC forwards information about the duplication to HealthCare Client Identity Management (HCIM) and MSP Rapid for further action. HCIM has duplicate detection, and all PHN merges are performed by HCIM staff. HCIM will notify PharmaNet of a merge when this happens to ensure the PHNs are merged in the PharmaNet tables. - Enter the PHN determined for use by the Help Desk on the local system.
HOW TO ENTER A PATIENT NAME INTO PHARMANET
When entering client name information into PharmaNet:
- Check the spelling of both the first and last name with the spelling on the client’s identification
- Check for spaces between parts of a name (e.g., van der Kamp vs. Vanderkamp)
- Confirm the spelling of abbreviations (e.g., Mac vs. Mc; Saint vs. St or Ste)
- Type carefully and verify that the name you have entered is accurate
- Do not add punctuation to a name field
- Do not add a title (e.g., Mr., Rev., Sr., Col.) in either name field
Section 3.4 Patients – Other Payers
General Policy Description
Clients may be covered by extended health insurance. Private insurers are companies, such as Pacific Blue Cross and Great West Life, that provide private, extended health insurance benefits that supplement the health care benefits of provincial programs such as MSP and PharmaCare.
PharmaCare is a provincial government reimbursement program and is not affiliated with any extended health insurance provider.
Community pharmacies have online access to some third-party insurers, such as Pacific Blue Cross and Assure. PharmaCare is not responsible for resolving any connection or adjudication problems that pharmacies may have with a third-party insurer. Clients may also have coverage through:
- A federal or other government insurer such as Veterans Affairs Canada, the Interim Federal Health Program, or ICBC
- An award for damages or settlement
Policy Details
PharmaCare does not provide claim adjudication results to other third-party payers.
Some third-party insurers (who provide private, extended health benefits) follow PharmaCare Special Authority (SA) requirements. Some third-party insurers provide coverage only if:
- PharmaCare has granted SA for the prescription
- PharmaCare SA was granted before the prescription was filled (i.e., they do not provide retroactive coverage)
All enquiries regarding retroactive coverage by a third-party insurer should be directed to the specific insurers, not PharmaCare.
WorkSafeBC/Insurance Corporation of BC (ICBC)
Eligible medication costs for WorkSafeBC clients must be claimed from WorkSafeBC, not PharmaCare.
Eligible medication costs for ICBC clients must be claimed from ICBC, not PharmaCare.
Federal coverage
PharmaCare is not the first payer for B.C. residents covered by the following federal plans:
- Veterans Affairs Canada (VAC)
- Canadian Forces
- Non-Insured Health Benefits (NIHB) Program
Many First Nations clients are covered by PharmaCare Plan W. They may also be eligible for federal coverage from Veterans Affairs Canada or the Canadian Forces.
First Nations individuals who are not eligible for Plan W continue to receive coverage through the NIHB.
The Patient Coordination of Benefits Table in PharmaNet, is loaded with PHNs for all B.C. residents covered by the above federal government agencies/programs.
PharmaCare does not cover drug costs for B.C. residents when they are full benefits under a federal plan. Only costs not reimbursed through federal coverage may be eligible for PharmaCare coverage.
Exceptions may be made to this policy under the Smoking Cessation Program.
Coverage for federal and provincial inmates and offenders
Federal offenders:
PharmaCare does not cover persons in custody in a federal institution as their drug costs are covered as part of the cost of their incarceration.
PharmaCare does cover federal offenders who have been released on day parole and approved to reside in a community-based residential facility.
Provincial offenders:
PharmaCare does not cover persons in custody in a provincial institution as their drug costs are covered as part of the cost of their incarceration.
PharmaCare does not cover provincial offenders who have been released on day parole but who must return to a correctional centre each night.
PharmaCare does cover provincial offenders who have been approved to reside in a community-based residential facility.
When an individual is released from a provincial corrections facility, the Provincial Health Services Authority is responsible for providing a short supply of medication to that individual, subject to some limitations.
For more information, refer to Health Care Services Manual of the Adult Custody Division, Section 7.16 – Medications Upon Release (PDF, 690KB) from the Adult Custody Division, Corrections Branch, Ministry of Public Safety and Solicitor General.
Awards for damages or settlements
If an individual has been, or will be, compensated for an injury, illness or other condition through an award for damages or settlement, PharmaCare does not cover the cost of any benefit used to treat that injury, illness, or other condition.
This policy does not apply to claims for clinical services or under the BC Smoking Cessation Program.
Ineligible costs
PharmaCare does not reimburse the cost of an eligible benefit if:
- The need for the benefit arose from an injury, illness or other condition alleged to have been caused by an act or omission of another person, and as a result of the allegations:
- a court has awarded damages to the beneficiary
- the beneficiary is entitled to compensation under a settlement agreement, or
- the beneficiary is entitled to compensation under a plan of private insurance or another legal instrument
Specifically, unless all damages or compensation payable have been fully exhausted, PharmaCare does not cover benefits when:
- A court has awarded damages to an individual for future care costs for drugs, devices, substances or related services
- A court has awarded damages to an individual for future care costs and the award does not distinguish between future care costs for drugs, devices, substances or related services and future care costs payable for other future care
- A court has awarded damages to an individual but the award does not specify the types of damages
- An individual is entitled to compensation under a settlement agreement for future care costs for drugs, devices, substances or related services
- An individual is entitled to compensation under a settlement agreement for future care costs that does not distinguish between future care costs for drugs, devices, substances or related services and future care costs payable for other future care, or
- An individual is entitled to compensation under a settlement agreement that does not specify the types of damages
Eligible costs
PharmaCare covers benefits required by an injury, illness or other condition when:
- A court has determined that no damages should be awarded to the individual.
- The individual is not entitled to any compensation under a settlement agreement.
- The individual has a potential claim for damages for the injury or illness, but liability has not yet been determined and no court has made an award for damages, nor has any settlement agreement for compensation been made.
- All damages or compensation payable for future care costs for drugs, devices, substances or related services or damages or compensation payable when the award or settlement does not specify the types of damages, have been fully exhausted.
Damage awards or compensation payable for types of damage other than future care and, where future care has been distinguished, other than future care for drugs, devices, substances or related services, does not affect a beneficiary’s entitlement to benefits for the injury or illness giving rise to the need for the benefit.
Proof of eligibility
PharmaCare may require an individual to provide details of a damages award or compensation or to provide a copy of the court judgment or relevant sections of the settlement agreement.
The individual may also be required to provide proof (through a notarized affidavit or other acceptable form) that the relevant portion of the damages award or compensation has been fully exhausted in order to be eligible for PharmaCare coverage for benefits for the treatment of the injury or illness in question.
Procedures for Pharmacies
Processing a prescription covered by WorkSafeBC or ICBC
- Identify the patient as a WorkSafeBC / ICBC client.
A WorkSafeBC / ICBC client should instruct the pharmacist that a prescription will be covered under a WorkSafeBC / ICBC claim. - Submit the intervention code "DE – Adjudicate to $0.00 as requested" in PharmaNet
Although a WorkSafeBC / ICBC claim adjudicates to $0.00, the medication information is recorded in the patient medication history. - Follow the relevant WorkSafeBC / ICBC claim procedures or collect payment from the patient.
Processing a federally-covered client’s prescription
- Enter the prescription as usual.
PharmaNet returns an error message. The PharmaCare-paid amount is zero, but the client’s medication history is updated. - Follow the relevant federal claim procedures or collect payment from the client.
Permanently changing federal coverage in PharmaNet
PharmaCare Help Desk staff can add or remove a PHN from the Patient Coordination of Benefits Table when necessary; for example, if a person has retired recently from the Canadian Forces.
Processing clients with federal coverage changes
- Call the PharmaCare Help Desk.
The Help Desk will change the client’s federal-coverage status in the Patient Coordination of Benefits Table. - Send the transaction to PharmaNet.
Medications not included in federal coverage
Sometimes federal coverage does not include a prescribed DIN/PIN, but PharmaCare does cover it. For example, a veteran may have certain pensionable injuries and VAC will pay only for drugs related to those injuries. In such situations, the PharmaCare Help Desk can temporarily change the client status.
If federal coverage may be available through special request, that process must be exhausted before contacting the Help Desk.
PharmaCare does not cover methadone for Non-Insured Health Benefits (NIHB) clients. For more information, refer to Section 8.8—Methadone Maintenance Payment Program.
To obtain temporary coverage for federal clients
- Call the PharmaCare Help Desk.
The Help Desk will change the client status in the Patient Coordination of Benefits Table from “federally covered” to “not federally covered.” - Send the transaction to PharmaNet (including any applicable dispensing fee).
After the transaction has been processed, the Help Desk will change the client status back to “federally covered.”
To process federally covered non-residents
- Enter the prescription in PharmaNet as usual (after assigning a PHN, if necessary).
The prescription information will be included in the patient medication history and the claim will adjudicate with the PharmaCare portion as $0.00, as it would for any other non–B.C. resident.
For more information on assigning a PHN for an out-of-province visitor, refer to Section 3.3—Pharmacist-assigned PHNs. - Follow the relevant federal claim procedures or collect payment from the client.
Section 3.5 Patients – Restricted Claimant Program
General Policy Description
Prescription medications are sometimes misused. Misused medications may include, but are not limited to, analgesics containing codeine, other narcotics, asthma medications and sleeping pills. The Restricted Claimant Program assists in reducing misuse by limiting coverage for certain clients to medications prescribed by a single prescriber and/or medications dispensed by a single pharmacy.
PharmaCare Audit oversees the program but day-to-day administration is handled by HIBC.
Policy Details
About client restrictions
Pharmacists, physicians and other healthcare professionals may contact PharmaCare to request restrictions for particular clients.
Requests can be processed by the PharmaCare Help Desk from 9 am to 4 pm, Monday through Friday, except for statutory holidays.
PharmaCare is responsible for determining whether to restrict client access to prescription medications that may be misused, by assigning one (or more) specific physician(s) and/or a single pharmacy in PharmaNet.
Potential restrictions are identified from routine monthly reports. The evaluation and decision process involves a review of the client’s claims history by a designated pharmacist. Such evaluations may also result from pharmacist/physician requests.
For each client to be restricted, PharmaCare takes one or both of the following two actions:
- Immediately adds a “Restricted Access” against the client PHN in PharmaNet.
This invalidates the PHN for all PharmaCare benefits (that is, PharmaCare will not contribute towards the cost of any prescription for the PHN) until information about the client’s physician and pharmacy is added. - Places a call to the client’s physician, to confirm that they are indeed that client’s physician.
If confirmed, the physician is recorded in the client’s PharmaNet file as the “physician of restriction.” If the client’s pharmacy is known, it is also recorded in PharmaNet. Or, the physician may provide an explanation that avoids a restriction.
A letter is sent to the “restricted claimant” advising of the restricted status and asking for the name of one physician and/or pharmacy, if this information has not already been obtained. Additional physicians, particularly specialists, will occasionally be added.
Lifting restrictions
Once a client has been classified as a “restricted claimant,” PharmaCare will only consider lifting the restriction:
- After the client has been restricted to that physician for at least six months, and
- Upon written request from the client’s physician.
The physician makes the request to lift the restriction in writing (by fax or letter) to PharmaCare. A designated pharmacist evaluates the request to lift a restriction by reviewing the client’s claims history. The physician is then notified of the decision by letter.
The lifting of a restriction takes effect immediately.
Emergency situations
In emergency situations, PharmaCare Help Desk staff may make one-day changes to the client’s designated doctor or pharmacy.
Exceptions are made only if the client is unable to see their own doctor or get to their own pharmacy and if not filling the prescription could result in serious harm to the client.
When a pharmacist calls to request a special exception, they will be asked a series of questions which Help Desk staff use to determine if financial coverage should be provided.
Help Desk staff will assess if there is a sufficiently good reason why:
- The client is unable to see their own doctor or go to their own pharmacy (e.g., doctor on vacation, pharmacy closed), and
- This prescription must be filled at this time (i.e., there is a serious health consideration rather than simply a request for an early refill).
The PharmaCare Help Desk is available Monday through Friday, 9 am to 4 pm. After-hours requests are subject to a more rigorous approval process because of the difficulty in following up with doctors.
Replacement of lost or stolen medications is not covered for clients in this program.
All special exceptions must be obtained before dispensing as there is no retroactive coverage.
Daytime calls to the PharmaCare Help Desk
During normal business hours (9 am to 4 pm, Monday through Friday), the PharmaCare Help Desk can answer calls from clients, pharmacists or physicians regarding restrictions. Or, pharmacists can choose Option 3 on the main menu to bypass the Help Desk and leave a message requesting a call back.
PharmaCare Help Desk staff may not disclose details of a patient’s previous prescriptions. Pharmacists can check the medication history returned by PharmaNet.
When PharmaNet returns a “Pharmacy not authorized” or “Physician not authorized” message, pharmacists may contact the PharmaCare Help Desk for more information.
Changes to the patient’s designated physician or pharmacy cannot be made automatically at a pharmacist's request. The decision is made, based on PharmaCare policy, by PharmaCare in consultation with Ministry of Health pharmacists and physicians.
After-hours calls to the PharmaCare Help Desk
After-hours, pharmacists may call the PharmaCare Help Desk to ask about restrictions. Help Desk staff follow PharmaCare policy/guidelines in handling all after-hours enquiries about restricted claimants.
Help Desk staff may not disclose details of a patient’s previous prescriptions. Pharmacists can check the medication history returned by PharmaNet.
If a pharmacist calls after hours, any change made by Help Desk staff (i.e., to the designated physician or pharmacy) will normally be in effect for no more than one business day. After-hours approval will not generally be given for drugs that are frequently misused.
If a restriction is changed temporarily to allow the processing of a specific prescription, only that prescription may be processed. Submission of additional prescriptions will be subject to audit and recovery.
To request a permanent change regarding a restricted claimant, the pharmacist will have to call during normal business hours and speak to Help Desk staff.
The Help Desk cannot make changes to restrictions when they are requested by a client after hours.
Section 3.6 Patients – With Out-of-Province/Country Prescriptions
General Policy Description
PharmaCare coverage is limited to medications prescribed by a practitioner licensed and practicing in British Columbia and dispensed at a B.C. pharmacy. Exceptions may be made for border community B.C. residents for whom the closest pharmacy is out-of-province.
Policy Details
Out-of-province prescriptions
As per the College of Pharmacists of BC, prescriptions issued in other provinces may be dispensed by a B.C. pharmacist when the pharmacist is sure that they are bona fide prescriptions.
For information on coverage of out-of-province prescriptions, refer to Section 1—Introduction to the BC PharmaCare Program.
Out-of-country prescriptions
Prescriptions issued in another country (including the USA) cannot be dispensed by a B.C. pharmacist. This is prohibited by the Canada Food and Drugs Act.
Anyone, whether or not a Canadian resident, who holds a prescription written by an out-of-country practitioner may have the prescription dispensed by a B.C. pharmacist only if the prescription has been either:
- Rewritten, on the practitioner’s own prescription pad, by a practitioner licensed to prescribe in a Canadian jurisdiction, or
- Co-signed by a Canadian practitioner
Section 3.7 Medical Practitioners – Prescriber IDs and Practitioner Reference IDs
General Policy Description
Each B.C. pharmacist, physician, surgeon, dentist, podiatrist, veterinarian, midwife, naturopath, optometrist and nurse practitioner on PharmaNet is assigned both a:
- Practitioner identification reference code (Pract ID Ref) that identifies the licensing body
- Practitioner identification number (also called a Practitioner ID, Pract ID, Prescriber ID or College ID), a unique number that identifies the individual practitioner, issued by the licensing body (e.g.,College of Physicians and Surgeons of British Columbia, College of Pharmacists of BC)
Policy Details
USE OF PRACTITIONER IDs
All prescriptions must be entered in PharmaNet with a valid Pract ID Ref and a Practitioner ID (i.e., the ID of the prescriber or, in the case of over‑the-counter medications, the pharmacist ID).
The Practitioner ID number must be used, not the MSP billing number.
OUT-OF-PROVINCE PRACTITIONER IDs
Practitioners from other provinces (except Alberta) are assigned generic Practitioner IDs; PharmaNet does not use the identification numbers issued by their respective colleges. Out-of-province practitioners, however, are always assigned Pract ID Ref codes to identify the relevant College.
Alberta physicians and surgeons have individual Practitioner IDs in PharmaNet, although there may be some exceptions; Alberta dentists, podiatrists and veterinarians are assigned a generic Practitioner ID.
Practitioner codes
For a claim to adjudicate in PharmaNet, a pharmacist must submit a valid, individual Practitioner ID number for a B.C. or Alberta practitioner, or the generic Practitioner ID for other out‑of‑province practitioners. For specialists, pharmacists must use the generic five-character Practitioner ID 99999.
- Refer to the list of Practitioner ID reference codes and Practitioner ID codes for Canadian prescribers
Using Practitioner IDs for non-prescription items
When submitting claims for non-prescription products, pharmacists may enter their Pharmacist ID in place of the Practitioner ID. If a pharmacist elects to use the Practitioner’s ID, the pharmacist must obtain authorization from the practitioner to dispense the item.
Pharmacists may also need to use their pharmacist ID in place of a Practitioner ID under other circumstances. For instance, the pharmacist ID would be used for:
- Claiming non-prescription items eligible for PharmaCare coverage
- Updating a patient medication history with a non-prescription item that is not eligible for PharmaCare coverage
- Dispensing emergency supplies of medication
- Claiming the administration of a publicly funded vaccine
- Claiming a clinical services fee
- Claiming medical supplies that are eligible for PharmaCare coverage such as insulin pumps
Device providers should use their own ID only for claiming supplies when they do not have current information on any practitioner responsible for the client’s care.
Procedure
PROCEDURES FOR PHARMACISTS
Finding the Practitioner ID for B.C./Alberta practitioner
- Search the local system for an existing practitioner entry. Once satisfied the prescriber is a legitimate practitioner, an entry for that prescriber may be created on the local system.
- Use the Prescriber Identification transaction (TIP) to search by surname only. If too many entries are returned, search by surname and first initial.
Use the same transaction to retrieve practitioner identity and practice information, entering the Practitioner ID number and Practitioner ID Reference code. - If you cannot locate the practitioner, call the PharmaCare Help Desk.
The Help Desk validates the practitioner name by repeating the name search or by contacting the appropriate college. If necessary, the practitioner can be added to PharmaNet, or the relevant college can be contacted (for B.C. physicians and surgeons only) to have the practitioner added.
Adding an Alberta practitioner to PharmaNet
To add an Alberta practitioner to PharmaNet, supply the following information to the PharmaCare Help Desk
- Practitioner name
- Practitioner address
- Practitioner gender
The Help Desk will ask you to use the generic Practitioner ID (99999) with the ID Reference code 81 in order to fill the prescription. It will contact the Alberta College of Physicians and Surgeons for confirmation so the physician can be added to PharmaNet.
Section 3.8 – (Removed)
Section 3.9 – Medical Practitioners - Authorized Prescribing
General Policy Description
A BC PharmaCare benefit must be prescribed by a licensed health professional with the appropriate prescribing authority.
Policy Details
BC PharmaCare benefits must be prescribed by a licensed health professional authorized to prescribe the benefit (or in the case of Plan W over-the-counter benefits, to recommend them).
In addition, if a drug, device, substance or related service is dispensed in a community pharmacy in B.C., the dispense must be recorded in PharmaNet by the dispensing pharmacist for it to be covered.
Prescribing must be in accordance with the bylaws and standards, terms and conditions (SLCs) of the prescriber’s health regulatory college, and all related federal and provincial statutes and regulations, such as:
- Controlled Drugs and Substances Act
- Food And Drugs Act
- Health Professions Act
- Health Professions and Occupations Act
- Pharmacy Operations and Drug Scheduling Act
- Pharmaceutical Services Act
PharmaCare covers prescriptions written in accordance with the relevant governing legislation, bylaws, and practice standards by:
- Physicians registered with the College of Physicians and Surgeons of British Columbia (CPSBC)
- Physician assistants (certified non-registrants of the College of Physicians and Surgeons of BC) as assigned and delegated by a supervising physician, as per 10(3) of the CPSBC bylaws
- Podiatric surgeons registered with the College of Physicians and Surgeons of British Columbia
- Midwives, nurse practitioners, registered nurses and registered psychiatric nurses registered with the British Columbia College of Nurses and Midwives (BCCNM)
- Dentists registered with the British Columbia College of Oral Health Professionals
- Optometrists registered with the College of Health and Care Professionals of BC
- Pharmacists registered with the College of Pharmacists of BC
- Naturopathic physicians registered with the College of Complementary Health Professionals of BC
PharmaNet does not have built-in restrictions that block the dispense of a particular prescription written by an authorized health professional. PharmaNet verifies that the prescriber is licensed to practice in B.C., but the prescriber and dispensing pharmacist are relied on to ensure prescriptions are valid.
The Ministry of Health and health regulators monitor both dispensing information and records of access to PharmaNet for compliance with regulatory requirements. PharmaCare may recover payments made for dispenses of invalid prescriptions.
Veterinary prescriptions
Veterinary prescriptions for animals are never a PharmaCare benefit, even if the same drug would be a benefit for a human. Any veterinary prescription dispensed in a community pharmacy must be entered in PharmaNet under the owner’s PHN and clearly identified as a dispense for an animal (an animal must never be assigned a PHN).
Pharmacists cannot accept prescriptions written by the pet owner’s doctor nor dispense prescriptions as an emergency supply or an adaptation under the pharmacist’s licence number.
- Read prescriptions for pets for how to record dispenses of veterinary prescriptions in PharmaNet
- Visit Section 5.3 for claim instructions in the rare event that a pharmacy is filling prescriptions for the same drug on the same day for both a pet and the pet’s owner
Section 3.10 – Medical Practitioners - Practicing Status and Practitioner Restrictions
General Policy Description
PharmaNet identifies:
- The practising status of a prescriber
- Any restrictions that preclude a practitioner from prescribing specific medications (e.g., narcotics or controlled or targeted substances)
This function identifies all members of the College of Physicians & Surgeons of British Columbia and the College of Dental Surgeons of British Columbia.
Practicing status and prescriber restriction data are supplied by the relevant college via direct, regular loads to the PharmaNet database. Questions regarding the application of practicing status and prescriber restrictions should be addressed to the relevant college.
Policy Details
PharmaNet will reject, without adjudication, any claims for prescriptions by non-practicing or unauthorized prescribers.
Practitioner status for midwives, podiatrists, nurse practitioners, optometrists, naturopaths, veterinarians and members of the College of Physicians & Surgeons of Alberta are not identified by this function. These prescribers are monitored through retrospective audits.
When a pharmacist searches for a practitioner using the Practitioner ID, PharmaNet provides the practitioner information and practising status.
When a pharmacist searches for a practitioner using the practitioner name, PharmaNet only provides information on practitioners who have practising status.
Section 3.11 – Medical Practitioners - BC Prescription Review Program
General Policy Description
The Prescription Review Program, which incorporates the former Controlled Prescription Program, periodically reviews drugs with a potential for misuse.
Under this program, information on certain drugs is downloaded automatically from the PharmaNet database to a drug information database maintained by the College of Physicians and Surgeons of BC (CPSBC). The College uses the information to review and identify potentially inappropriate prescribing, multi-doctoring, drug diversion, and opportunities for physician education.
Policy Details
Drugs requiring use of a duplicate prescription
The Prescription Review Program, in place since 1990, maintains a list of opioids for which a duplicate prescription is required.
The CPSBC requires that one copy of the duplicate form be retained by the prescribing physician and the other copy be retained by the pharmacy.
Information on the drugs requiring use of the duplicate form, including a list of the drugs, is available in the Prescription Review Program section of the CPSBC website.
Non-duplicate opioids
The Prescription Review Program also reviews the clinical use of non-duplicate opioids and benzodiazepines.
A special prescription pad is not required for these drugs.
Section 3.12 – Drug and Product Identification Numbers
General Policy Description
Health Canada assigns a Drug Identification Number (DIN) to all drug products.
If Health Canada has not assigned a DIN for a product, PharmaCare assigns a product identification number (PIN) that allows claims for the product to be adjudicated by PharmaNet.
When Health Canada issues a licence for a natural health product—after assessing and finding it to be safe, effective, and of high quality under the recommended conditions of use—they assign the product an eight-digit Natural Product Number (NPN) or Homeopathic Medicine Number (DIN-HM), which can be found on the label.
PharmaCare must assign a PIN for compounded medications, investigational drugs, natural health products that have not been assigned an NPN, and non‑pharmaceuticals such as blood glucose monitors and test strips, prosthetics, orthotics, and ostomy supplies.
Policy Details
Pharmacists and medical device providers must use the appropriate PIN, found in the lists linked below.
Drug Use Evaluation (DUE) results are not returned for claims using PINs.
Procedures
Identifying a PIN
Product Identification Numbers (PINs) are used when no DIN is assigned to a product.
PharmaCare publishes lists of PINs for various categories of products on its website.
All PINs: Product Identification Numbers (PINs)
Individual PINS:
- Allergy serums
- Compounded prescription products
- Cystic fibrosis products
- Diabetes items:
- blood glucose test strips (BGTS)
- continuous glucose monitors (CGM)
- flash glucose monitors (FGM)
- insulin pumps
- insulin pump supplies
- needles and syringes
- Miscellaneous products
- Nicotine replacement therapy products and smoking cessation prescription drugs
- Opioid agonist treatment
- Ostomy supplies
- Prosthetics and orthotics
- Publicly funded vaccines
Need help determining the correct PIN? Call the PharmaCare Help Desk.
Section 3.13 – Correct Quantities
General Policy Description
Accurate quantity reporting is critical to efficient and accurate adjudication of claims.
Policy Details
Section 5.5—Correct Quantities Policy explains that entering the correct dispensed quantity (e.g., number of syringes, volume in millimetres) in PharmaNet ensures the efficient and accurate adjudication of claims.
The Correct quantities for Pharmacare claims web page and the BC PharmaCare Formulary Search provide the quantity to enter in PharmaNet for specific products.
Pharmacist best practices
Health Canada assigns one drug identification number (DIN) per drug entity. This calls for attention for drugs marketed in unit dose syringes, vials or packs of the same concentration but of varying volumes.
Practitioners, especially emergency department physicians and hospital pharmacists, rely on the information in the PharmaNet profile when determining what doses the patient has already had and what doses to re-prescribe.
Pharmacist best practice includes:
- Document in the SIG field the exact dose that was dispensed
- Ensure doses are confirmed by a third party (e.g., patient, prescriber, hospital or clinic) before new orders are written and filled
Procedures
Visit Correct quantities for PharmaCare claims to find the correct quantities to enter in PhamaNet.
The BC PharmaCare Formulary Search may also be helpful in determining the unit of measure used for a particular product.
If you are still unsure which unit of measure to submit or are having trouble entering a decimal, contact the PharmaCare Help Desk
Section 3.14 – Drug Use Evaluation (DUE)
General Policy Description
PharmaNet captures and adjudicates all prescriptions dispensed in all community pharmacies and hospital outpatient pharmacies in B.C. Once a transaction is completed, PharmaNet returns a complete patient medication history, drug use evaluation (DUE) and adjudication results.
Policy Details
DUE checks
The last 14 months, or the last 15 dispenses within the past 14 months, of all prescriptions dispensed (i.e., a client's medication history) in community pharmacies and hospital outpatient pharmacies in B.C. is available in PharmaNet.
PharmanNet's DUE function provides pharmacists with information for assessing patient therapy.
PharmaNet performs four types of DUE checks, using drug information and clinical modules from First Databank (FDB).
The prescription being dispensed is compared to active prescriptions in the medication history to assess:
- Drug-to-drug interactions
- Drug-to-prior adverse reactions
- Duplicate therapy/ingredients
- Dose too high/too low
DUE and opioid agonist treatment
Opioid agonist treatment (OAT) is frequently not included in DUE checks for OATs dispensed under PINs, as including it causes an excessive number of Duplicate Ingredient/Duplicate Therapy messages.
OATs dispensed under DINs (e.g., buprenorphine-naloxone) are included in DUE checks.
Pharmacists dispensing prescriptions to patients on OAT should be aware of potential drug interactions for OAT drugs, and be prepared to recommend appropriate monitoring and management strategies to physicians and patients.
For information on potential drug interactions, refer to CPBC's Professional Practice Policy (PPP) 66 Guide.
Section 3.15 – Drug Monograph Information
General Policy Description
The following monographs are available on PharmaNet:
- Patient education monographs
- Counselling message monographs
- Drug-to-drug interaction monographs, if implemented by the pharmacy software vendor
Policy Details
Patient education monographs
Drug monograph information for patients is supplied by First Databank (FDB) and is sometimes augmented by the CPBC. Optional access to generic-equivalent information is also available through the same PharmaNet transaction. These monographs can be printed for the client.
- Patient education monographs assist pharmacists in counselling patients about proper use and side effects of medications. The information is presented in language that is easy to understand
- The education long monograph explains the reasons for taking the medication, how it should be taken, potential side effects, and how to handle missed doses. It also details precautions when taking the medication, potential drug interactions, and proper storage
Counselling message monographs
Counseling message monographs are available in pairs—one for the pharmacist and the other for the patient. Each pair is rated on the importance of the information to the patient. All messages can be printed for the client.
- Counselling professional monographs provide detailed explanations of proper use, side effects and other information
- Counselling patient monographs provide equivalent information to the counselling professional monograph, but in simpler language
Drug-to-drug interaction monographs
A drug-to-drug interaction monograph for all possible drug-to-drug interactions for a specific medication can be accessed by entering a DIN.
A drug-to-drug interaction monograph for two specific medications can be accessed by entering two DINs.
Section 3.16 – Claim Reversals
General Policy Description
Under certain circumstances, it is necessary to reverse a claim. Reversing a claim modifies the original claim status on the client's medication and claims history in PharmaNet to “reversed” and results in a billing correction.
Policy Details
Policy regarding claims reversals
Reversing a claim modifies the original claim on the client’s medication and claims history in PharmaNet to “reversed” and automatically causes a billing correction.
If an error related to a DIN, PIN, drug name, strength, quantity, practitioner ID or instructions for use is identified, the provider must reverse the claim in PharmaNet, any third-party insurer system, and the local system. The provider should then enter the corrected claim.
Reasons for reversing a claim may include the following:
- The prescription was:
- not picked up by the patient
- dispensed, but the quantity was changed at a later date
- dispensed under the wrong PHN
- changed/cancelled in consultation with the physician
- A data entry error
- A network problem required the claim to be reversed and re-entered (these are called “network reversals”)
Important: A "Reversed" status code does not necessarily mean the drug was ever prescribed for, or taken by, the patient.
As per CPBC:
- Only the refill authorization field may be modified without reversing the prescription
- Modifying the prescription history for a dispensed prescription in the local software is prohibited
- Medications not picked up by the patient must be reversed and returned to stock within 30 days of the dispensing date.
- Reversals due to billing adjustments must be made within 120 days of the dispensing date
Learn more at Pharmacy Operations and Drug Scheduling Act Bylaw 35 (3)–Data Collection, Transmission of and Access to PharmaNet Data (PDF, 350KB).
All prescriptions reversed on PharmaNet, except reversals using the intervention code RE — Claim reversed, data entry error will appear on the patient’s medication history.
Providers may reverse a claim up to 120 days after the dispensing date.
Procedures
ROUTINE REVERSALS
Backdating re-entered prescriptions
All corrections to the medication history require that the prescription(s) be reversed and re-entered with the correct information, using the same date as the original prescription, not the date of correction. It is imperative that patient histories show the correct medications with the correct dispensing dates.
Backdating claims in PharmaNet is only permitted only correcting a previously adjudicated claim, not to alter adjudication results.
For further information, refer to Potentially Fraudulent Reversals.
Reversals affecting deductibles
Billing corrections caused by reversals may affect client deductibles. A client who has previously met the annual deductible may fall below the deductible due to a claim reversal. If so, the client must pay some or all of the cost of later claims.
Intervention codes for reversals
| Code | Meaning |
|---|---|
| RU | Claim reversed, not called for (not picked up) |
| RE | Claim reversed, data entry error |
| RC | Prescription cancelled by physician |
| RR | Prescription refused by patient |
| NR | Non-returnable drug reimbursement |
| UB | Consulted prescriber and changed dose |
| UC | Consulted prescriber and changed instructions for use |
| UD | Consulted prescriber and changed drug |
| UE | Consulted prescriber and changed quantity |
Reversing claims after 121 days
To request the reversal of a prescription after 121 days, providers must contact the PharmaCare Help Desk.
For reversals over 180 days, please contact PharmaNet Data Quality Services.
Network reversals of claims
PharmaNet data integrity errors can require the provider or the PharmaNet Data Quality Services team to reverse a claim.
A claim in PharmaNet generates a record in the medication history and one in the claims history. If a network problem occurs, the system may complete half the process, creating a record in the claims history without a corresponding record in the medication history, or vice versa. These are referred to as “orphan” records and must be corrected so that medication histories and claims histories are complete. When an orphan record is detected, PharmaNet will send an error message to the provider.
The Patient Coordination of Benefits (PATCoB) table in PharmaNet is loaded with PHNs for all B.C. residents whose drug costs are covered by one of several federal government agencies or programs. If a PHN is in the PATCoB table because the patient has federal coverage, a record is created only in the medication history. This record is not considered an orphan record. Such PHNs will return an error message on the claim.
Eliminating an orphan record
To eliminate an orphan record, contact your software vendor or the PharmaCare Help Desk.
Potentially fraudulent reversals
Providers must not reverse and re-submit claims on a different date so that a client receives PharmaCare coverage to which they were not entitled when the item was actually dispensed.
PharmaCare Audit can examine claims histories for evidence of this activity.
Reversing claims dispensed under the wrong PHN
Occasionally, a provider may discover that a previous claim was dispensed under the wrong PHN. These cases may come to light when a patient expresses surprise at having to pay “out of pocket.”
Some local software allows the reversal and correction of wrong PHN on claims; however, you may need to contact PharmaNet Data Quality Services.
To correct a "wrong PHN" prescription within 120 days
- Reverse the claim using the incorrect PHN.
- Correct the PHN in your local system.
- Re-send the claim to PharmaNet with the original dispensing date.
Your software vendor or PharmaNet Data Quality Services can help with reversing a claim.
To correct a "wrong PHN" claim after 121 days
Contact PharmaNet Data Quality Services. These claims cannot be reversed by providers or by PharmaCare Help Desk staff.
Medical Assistance in Dying claim reversals
This policy applies to Medical Assistance in Dying (MAiD) claims.
All claims for medications in a MAiD kit, including the Clinical Services Fee ($100 for primary and secondary IV drug regimen, $60 for primary oral drug regimen and secondary IV regimen) must be reversed when the MAiD kit has not yet left the pharmacy, and the patient:
- Is ineligible after being assessed as eligible
- Has died from another cause
- Has withdrawn their request
If the withdrawal happens after the MAiD kit left the pharmacy, and the unused MAiD kit is returned, the pharmacy should not reverse any claims; the pharmacy is eligible for a dispensing fee, cost of the medications used in the MAiD kit, and the appropriate Clinical Services Fee.
Unused medications can only be returned to stock if the exemption is met in section 5 of Dispensing Drugs for the Purpose of Medical Assistance in Dying Standards, Limits and Conditions.
HANDLING CLAIM REVERSALS WHEN CHANGING OR UPGRADING LOCAL SOFTWARE
When vendors install new software or upgrade an existing product, they perform a software conversion. In this conversion, existing data files from the old software are transferred to the new system. After a software conversion, claims submitted using the old software cannot be reversed with the new software. Providers are advised to reverse claims before a software conversion—and to talk to their software vendor before the conversion to ensure there is enought time.
Before the conversion, reverse every claim for an item that has not yet been picked up, including those you expect the patient to pick up. After the conversion is complete, re-submit each claim using your new software.
Section 3.17 – Prescription Discontinuations
General Policy Description
A prescription should be discontinued if a patient has stopped using a medication previously dispensed.
Discontinuing a prescription changes the status in PharmaNet to “discontinued” and changes the expiry date of the prescription so that the prescription will not be included in drug use evaluation (DUE) checks.
A discontinuation does not trigger a billing correction, and the medication remains in the patient medication history in PharmaNet.
As per the College of Pharmacists of BC, a prescription may be discontinued only:
- By the pharmacy that originally dispensed it, and
- If the original prescription is recorded in PharmaNet
Section 3.18 – Claims for Drug Cost Exceeding $9,999.99
General Policy Description
The maximum drug cost that can be entered in PharmaNet is $9,999.99. However, drug claims that exceed this amount are not uncommon. This section explains how these claims should be submitted.
Policy Details
Claims in excess of $9,999.99 must be split, with the drug cost, dispensed quantity, and days' supply divided proportionately between the claims.
Dispensing fees may not be split.
Procedure
To ensure correct adjudication of claims exceeding the maximum:
- Split the claim and submit as separate claims of less than $9,999.99
- Divide the drug cost in proportion to the dispensed quantity entered for each claim
- Pro-rate the days' supply between the claims in proportion to the dispensed quantity entered for each claim (example below)
- Do not split the dispensing fee; include it in only one of the claims, entering a $0 dispensing fee on the remaining claims
- Enter the intervention code MP for all claims except the first claim
When you must submit multiple claims due to drug cost exceeding $9,999.99, you are required to ensure any portion of the days’ supply claimed in excess of the PharmaCare maximum for the drug (i.e., 30 or 100 days, as applicable) is entered into PharmaNet with the intervention code DE—Adjudicate to $0.00 as requested.
Sample of submission of a claim in excess of $9,999.99
| Field | Claim 1 | Claim 2 | Claim 3 |
|---|---|---|---|
| Dispensed quantity | 10 | 10 | 8 |
| Days' supply | 10 | 10 | 8 |
| Drug cost | $8,571.43 | $8,571.43 | $6,857.14 |
| Dispensing fee | Yes (usual fee claimed) | No (zeroed out) | No (zeroed out) |
| Intervention code | N/A | MP | MP |
Section 3.19 – Recording Adverse Drug Reaction and Allergy Information in PharmaNet
General Policy Description
Whenever a pharmacist is made aware that a client has had an adverse drug reaction that will affect their future medical care (including allergies to prescription drugs, non-prescription medications, or natural health products), the pharmacist must update the client’s medication profile in both the local system and PharmaNet.
Policy Details
If an adverse drug reaction will affect a client's future medical care, a pharmacist must update the patient’s medication profile in both the local system and PharmaNet.
Pharmacy software may not automatically update a patient’s profile in PharmaNet. Pharmacists can contact their software vendor to determine if their software requires them to manually trigger the upload to PharmaNet.
Procedure
Entering an adverse drug reaction
- Use the Patient Profile Information Update—TPI function.
- Enter the required information in the Add Adverse Drug Reaction screen, as described below:
| Field name | Mandatory | Information details |
|---|---|---|
|
DIN |
Yes |
Drug DIN, PIN, or NPN —refer to information below to determine the correct information to enter |
|
Drug name |
Maybe |
Drug's generic name. Some software auto-completes the drug name when the DIN is entered. |
|
Reported by |
Yes |
Person who reported the reaction to you, i.e., client or family member, pharmacist, physician, BC Drug and Poison Information Centre |
|
Date reported |
Yes |
Date on which the adverse reaction was reported to you |
|
Comments |
No |
Details about the adverse reaction or allergy (maximum length that can be uploaded to PharmaNet is 80 characters) |
|
Practitioner ID reference code |
Yes, if comments are included |
Your information as the health care provider entering the information |
|
Practitioner ID |
Yes, if comments are included |
Your information as the health care provider entering the information |
|
Date entered |
Yes, if comments are included |
Date the adverse reaction was entered in PharmaNet. Your software may not display this field if it automatically fills in the date |
For non-prescription or natural health products
Certain non-prescription or natural health products do not have a DIN; PharmaCare assigns a PIN to such products.
To decide which identifier to use:
- If a DIN exists for a product, enter the DIN
- If there is no DIN, enter the product's unique PIN
- If there no unique PIN for a product, enter the appropriate Miscellaneous PIN and provide product details in the Comments field
For compounded products
When a client has an adverse reaction to a product compounded with multiple ingredients that have DINs, create an adverse reaction record for each DIN in the product, unless the client knows the particular ingredient they are allergic to.
3. After recording the reaction in your local system, follow your software's procedures for uploading the entry to PharmaNet.
Entering general allergy information
When someone reports an allergy that is not specific to a particular product, enter the information in the Clinical Conditions screen of the Patient Profile Information Update—TPI function.
Removing adverse drug reaction information from PharmaNet
If you (or a client) identify inappropriate or incorrect information in the adverse drug reaction field, submit a Request to Inactivate Adverse Reaction/Clinical Condition in PharmaNet Profile (HLTH 5550) (PDF, 930KB).
3.20 Veterinary prescriptions
General Policy Description
Veterinary prescriptions are never a PharmaCare benefit. Every veterinary dispense must be recorded in PharmaNet.
Policy details
Veterinary prescriptions for animals are never a PharmaCare benefit, even if the same drug would be a benefit for a human.
Any veterinary prescription dispensed in a community pharmacy must be:
- Written by a veterinarian – physicians are not permitted to prescribe drugs for animals
- Entered in PharmaNet under the owner’s PHN, and
- Clearly identified as a dispense for an animal
A PHN must never be assigned to an animal.
To prevent a drug for an animal from adjudicating in PharmaNet as a PharmaCare benefit:
- Use the veterinarian’s licence number as the Practitioner ID
- Use V9 as the reference code for the College of Veterinarians of BC
This ensures the drug does not appear on the pet owner’s record as a dispense for them, which would affect Drug Use Evaluation (DUE) results.
Failure to properly process veterinary medications in PharmaNet may result in inappropriate PharmaCare coverage and could have serious implications for client safety.
Adaptation and emergency supplies
Pharmacists cannot dispense prescriptions for animals as an emergency supply or an adaptation under the pharmacist’s licence number.
Claims for the same drug on the same day for a client and their pet
In the rare situation in which a pharmacy fills a prescription for the same drug on the same day for both a pet and a pet owner:
- Submit the claim for the client (pet owner) first to ensure the pet owner's medication profile is accurate
- If the claim for the pet has already been processed, submit the client claim with the intervention code UF – patient gave adequate explanation
If you forget to enter the UF intervention code and the claim is rejected, try reversing the claim first and reprocessing it with the UF intervention code. Contact the PharmaNet Data Quality Services Team if you need help correcting the problem.
Section 3.21 Office-Use Medications
General Policy Description
Once enrolled as a PharmaCare provider, every pharmacy is assigned a unique office-use medication (O-Med) PHN and keyword. The O-Med PHN must be entered in PharmaNet when a pharmacy dispenses medications to practitioners to provide to patients in their clinic or office. Hospital outpatient pharmacies must request an O-Med PHN.
Policy details
O-Med PHN
Each pharmacy is assigned a unique office-use medication (O-Med) PHN when they enrol as a PharmaCare provider. A pharmacy must enter the O-Med PHN in PharmaNet when dispensing medications for a practitioner (physician, dentist, veterinarian or midwife) to provide to patients in their clinic or office.
O-Med PHNs cannot be used for stock transfers between pharmacies. Stock transfer functions are unique to each pharmacy’s local software. Pharmacies should direct any questions to their software vendor rather than to the PharmaCare Help Desk.
Pharmacies should not use the O-Med PHN when dispensing a prescription to a practitioner for the practitioner’s personal use. They should use the practitioner’s PHN instead.
O-Med PHNs cannot be shared between stores.
If a pharmacy was not assigned, does not know, or has lost its O-Med PHN, the pharmacy may call the PharmaCare Help Desk to get their O-Med PHN.
Procedures
O-Med patient record
Before dispensing O-Meds, set up patient record(s) using your O-Med PHN.
- If your software allows multiple records under the same PHN, set up an individual patient record for each practitioner you dispense O-Meds to
- If your software does not allow multiple records under the same PHN, set up only one new patient record
Dispensing O-Meds
When entering PharmaNet claims for O-Meds, pharmacists should enter:
- The pharmacy’s O-Med PHN and keyword
- The date of dispense
- The correct quantity and practitioner number
If dispensing the same DIN more than once on the same day, use the intervention code UA to prevent receiving a “duplicate” message.
Once dispensed, PharmaNet returns:
- An “E1 Host Processing” error, which indicates that the claim has not been adjudicated, and no costs will accumulate toward a patient deductible
- A Drug Use Evaluation (DUE) message
Reversing O-Med transaction
O-Med transactions are reversed in the same way as other transactions. To reverse an O-Med transaction, use the O-Med PHN, the keyword, and the intervention code RE.
For more information, visit Claims Reversals
O-Med medication history
A pharmacy may review its O-Med dispensing history in PharmaNet by using:
- Last 15 Prescriptions transaction (TRR) or
- PharmaNet Record Request transaction (TRP) for history beyond the previous 15 entries (this transaction may take some time)
Changing O-Med keyword
To change the keyword associated with an O-Med PHN, the pharmacy should call the PharmaCare Help Desk. The keyword can’t be changed through the pharmacy’s local system.
Questions about O-Med medication histories should be directed to the Ministry of Health’s PharmaNet Data Quality Services team at 1-844-660-3200.

4 - Offline (Manual) Claims
Section 4.1 – Claims by Offline and Out-of-Province Providers
General Policy Description
Some PharmaCare providers are not connected to PharmaNet. They or their clients must submit claims to PharmaCare manually. These PharmaCare providers are:
- Offline providers: Non-pharmacy providers in B.C. who opt out of connecting to PharmaNet
- Out-of-province (OOP) providers: Providers outside of B.C. who cannot connect to PharmaNet; connection to PharmaNet must be within B.C.
Policy Details
Non-pharmacy providers in B.C. may opt out of connecting to PharmaNet. These are offline providers.
Some out-of-province (OOP) providers can enrol in the PharmaCare program in order to submit claims to PharmaCare or enable their patients to be reimbursed by PharmaCare but they cannot access PharmaNet since access to PharmaNet is restricted to sites in B.C.
Offline and OOP providers may submit manual claims to PharmaCare (i.e., by mail, fax or courier) for benefits provided to B.C. residents through PharmaCare’s 100% coverage plans (Plans C, D, F, G, W and Z).
Offline and OOP providers should not submit manual claims to PharmaCare for benefits provided to B.C. residents under Fair PharmaCare (Plan I). These beneficiaries may submit claims using the Manual Patient Claims process detailed in Section 4.2—Manual Patient Claims (providers should support their claims by providing them with a receipt showing the amount was paid in full).
OOP sites enrolling as providers are required to sign a declaration. By signing the declaration, OOP providers agree to serve the residents of B.C., to be bound by the laws of the Province of B.C. and to have any court proceedings related to their enrolment in PharmaCare conducted in B.C.
If enrolment is approved, the site becomes a PharmaCare provider, but is not connected to PharmaNet.
OOP providers are subject to the same policies and procedures as in-province providers.
Payment is not made for B.C. residents visiting an OOP community; payment is provided only because the OOP provider is closer or more accessible to a B.C. resident’s home than the nearest B.C. provider. However, in some limited circumstances, payment is also made because an OOP provider has been approved to sell certain products to B.C. residents that will be covered by the PharmaCare program.
Offline and OOP providers should note that an individual ceases to qualify for PharmaCare coverage on the day they leave B.C. to reside elsewhere, even though Medical Services Plan (MSP) coverage may continue.
Offline and OOP providers may opt out of PharmaCare at any time, but if they do so, their clients will not be reimbursed by PharmaCare for purchases made at their site.
All claims by providers are subject to PharmaCare Audit.
Processing manual claims
PharmaCare adjudicates manual claims using the PharmaNet system.
Several claims can be submitted manually at the same time under one invoice. If a manual claim is accepted, payment for the PharmaCare portion is issued by the Ministry of Finance, and a statement (the "remittance advice") is sent by PharmaCare to the provider. The remittance advice itemizes the processed claims.
Documentation submitted with a claim that is accepted is not returned.
Claims that are accepted and processed are returned to the provider with a remittance advice. Claims that are not processed because they are incomplete or incorrect, are returned to the provider without a remittance advice.
For more information, please refer to Incomplete/Incorrect Claims below.
Direct deposit payments
The Ministry of Finance issues payments to providers for accepted claims. To receive payment by electronic funds transfer, complete a British Columbia Government Direct Deposit Application. Submit the application and an original void cheque to:
PharmaCare Information Support
PO Box 9655 Stn Prov Govt
Victoria BC V8W 9P2
The Direct Deposit Application is in the PharmaCare Welcome Package sent to new providers, and additional copies are available from Health Insurance BC (HIBC) Info Support at informationsupport@hibc.gov.bc.ca.
Manual claims by providers
All manual provider claims must include:
- A completed PharmaCare Claim Form (HLTH 5336)
- A record of the details of each individual product dispensed
- A work order/invoice, with costs itemized
- A completed PharmaCare Prescription Invoice (HLTH 5335), which summarizes the total number of claims and total dollar value of the invoice submitted
Up to 100 claim forms may be submitted with one Prescription Invoice.
Completing the PharmaCare Claim Form (HLTH 5336)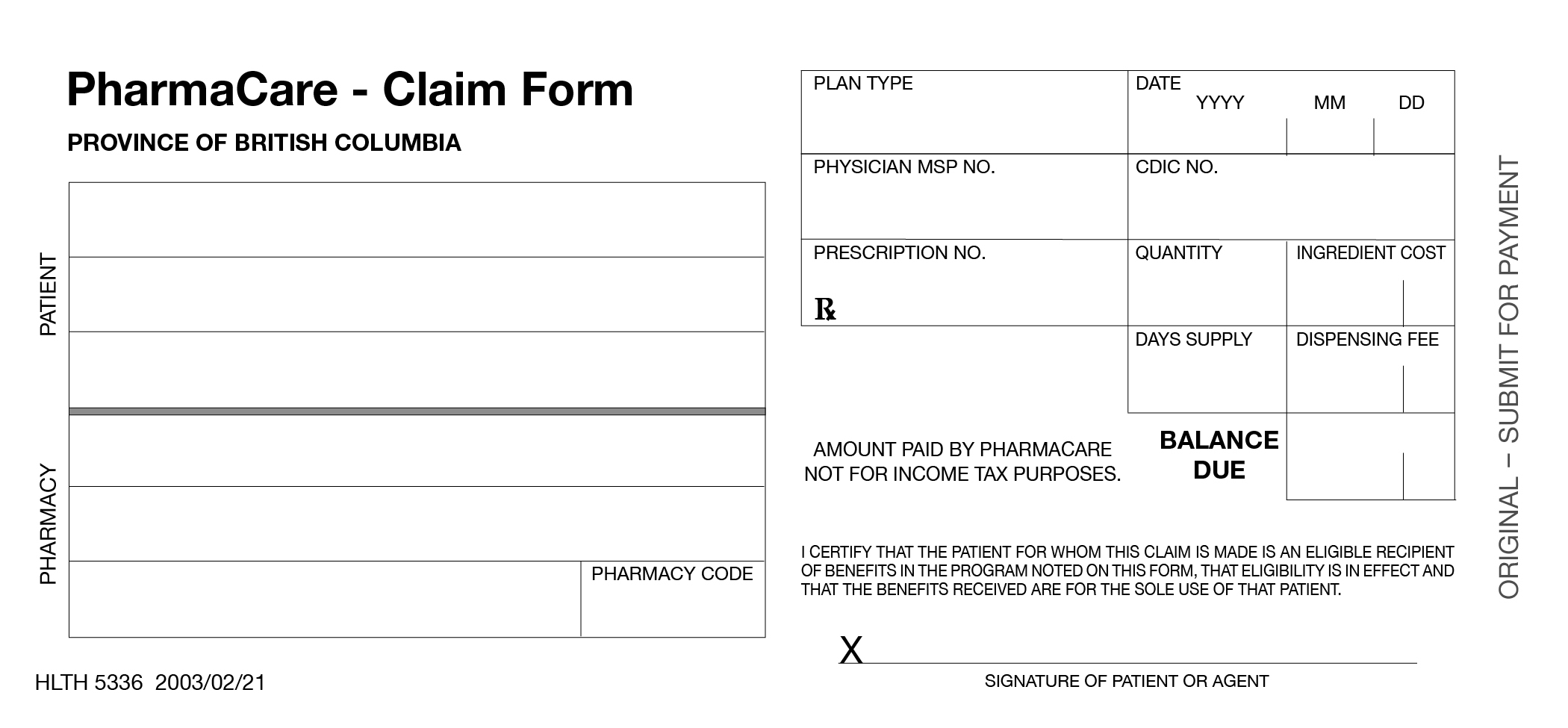
Ensure that all the following information is provided and that the correct units of measure are used. If unsure of the unit of measure to use for a particular product, consult the Correct Quantities list.
- Complete a PharmaCare Claim Form for each product or service (even if multiple products/services are provided for the same patient), recording:
- Patient's name and Personal Health Number (PHN)
- Date (YYY/MM/DD) that the device or service was provided
- Name of the provider to whom payment should be sent
- Provider's Site ID
- PharmaCare plan (C, D, F, G, W, or Z)
- Prescribing physician's MSP billing number, or prescribing nurses’ college ID
- Provider's work order or invoice number
- Full quantity of the drug or product covered by the claim. See Correct Quantities
- CDIC No. – the DIN (for drugs) or the PIN (for compounds or products). Refer to the PIN's list at Information for Pharmacies and Information for Device Providers
- Dispensing fee (up to the PharmaCare maximum)
- Ingredient cost (up to maximum covered by PharmaCare). See Sections 5.6—5.22
- Total claimed – total $ of Dispensing Fee and Ingredient Cost
- Balance due – same as total claimed
- Patient/agent's signature (an agent may be the parent of a child patient or someone with Power of Attorney for a patient)
2. Submit the top copy of each PharmaCare Claim Form to PharmaCare with supporting documents such as work orders or invoices (up to 100 Claim Forms can be sent in with each Prescription Invoice). Give the second copy to the patient. Retain the third copy for your records.
PharmaCare Prescription Invoice (HLTH 5335)
 Complete the invoice, with the following information:
Complete the invoice, with the following information:
- Pharmacy ID - provider's name and address
- Date of the invoice (YYYY/MM/DD)
- Total number of claims being submitted with the invoice
- Provider's Site ID
- Total amount invoiced - total $ amount for all the claims covered by this invoice
- Signature - Provider's authorized representative's signature
- Position or title of the person who has signed the invoice
Submit the Prescription Invoice with up to 100 claim forms and associated documents by mail to:
PharmaCare
PO Box 9655 Stn Prov Govt
Victoria BC V8W 9P2
Retain the second copy of the invoice (labelled PHARMACY) for your records.
Forms may be couriered to:
PharmaCare
Health Insurance BC
2261 Keating Cross Road; Block B - Unit #200
Saanichton, BC V8M 2A5
Processed claims are returned with the remittance advice. PharmaCare returns claims that cannot be processed to the provider for correction or completion. PharmaCare deducts the amount of the incomplete or incorrect claim(s) from the invoice total. The Prescription Invoice is not returned.
If an incomplete or incorrect claim is returned to you:
- Correct or complete the original form; do not submit a new claim form.
- Ensure the date on the claim form is the date the client received the product or service.
- Include the $ amount of the corrected claim on a new PharmaCare Prescription Invoice.
- Submit the Claim Form and Prescription Invoice to HIBC.
If the claim is rejected due to patient ineligibility or because the product is not a benefit, the claim form is returned. These claims should not be re-submitted.
Section 4.2 – Manual Patient Claims
General Policy Description
B.C. residents may request reimbursement for items they paid for that are eligible for PharmaCare coverage. This includes devices that the provider did not submit a claim directly to PharmaCare for, benefits from PharmaCare-approved border pharmacies in Alberta, offline medical device suppliers (e.g., ostomy supplies), and benefits purchased out of province by clients of the First Nations Health Authority.
Policy Details
B.C. residents can request reimbursement for PharmaCare benefit items they purchased themselves. Claims can be submitted any time during the year they're purchased and as late as March 31 of the following year. One claim submission can include up to 100 eligible purchases.
Process for claims other than prostheses and orthoses
The patient writes a letter that explains why they paid for items that were eligible for PharmaCare coverage. This could be, for example, because they bought them at a PharmaCare-approved pharmacy across the border in Alberta.
The patient sends the letter and store receipts for the items purchased to PharmaCare by fax or mail (see below). The letter must include the patient’s full name, birth date, personal health number (PHN) and address. Submitted receipts/invoices must show itemized costs and include the provider’s name and address.
If requested by the patient, the provider can mail or fax the letter and receipts/paid invoice to PharmaCare.
Process for prostheses and orthoses
In most cases, the device supplier will submit the patient’s paid invoice and the approved PharmaCare application form (e.g., HLTH 5400, HLTH 5404).
If a patient wishes to submit the claim, they will request that the supplier submit the PharmaCare invoice on their behalf, and the patient will mail or fax the receipt(s) and any other supporting documents to PharmaCare.
A Fair PharmaCare client may submit a claim for a prosthetic or orthotic device or service directly to PharmaCare only if they paid the provider the entire cost of the device or service. Reimbursement will depend on the patient’s Fair PharmaCare rules (i.e., deductible, family maximum).
Send claims to:
PharmaCare
PO Box 9655 Stn Prov Govt
Victoria BC V8W 9P2
OR
Fax: 250-405-3587 — All receipts must be clearly visible and “PRIVATE FAX” must be written on the first page if the patient wishes to receive return correspondence by fax.
After the claim is processed
PharmaCare will return all receipts that are sent by mail, along with a PharmaCare Remittance Statement showing the items and amounts reimbursed and a cheque, if applicable. A patient covered by a third-party insurer may use the returned receipts and statement to submit a claim to that insurer.
Note that if Fair PharmaCare is the patient’s benefits plan and they have not met their deductible and family maximum, PharmaCare may reimburse only some (or none) of the amount claimed.

5 – Pricing Policies and Product Reimbursement
Section 5.1 – Maximum Days' Supply Policy
General Policy Description
The Maximum Days' Supply policy limits PharmaCare coverage of drugs to fills of either 30 or 100 days to address concerns over public safety and drug wastage.
Policy Details
To ensure the appropriate adjudication of claims, pharmacies must enter in PharmaNet the accurate days' supply for the drug dispensed.
Claims with an inaccurate days' supply may be subject to audit and recovery.
100-Day Maximum Days' Supply
PharmaCare limits coverage to a maximum 100-day supply for repeat fills for long-term maintenance drugs.
30-Day Maximum Days' Supply
PharmaCare limits coverage to a maximum 30-day supply for:
- All fills for short-term drugs and other drugs assigned a 30-day supply maximum by PharmaCare, and
- First fills for long-term maintenance drugs to ensure the drug is effective and tolerated by the patient
Short-term drugs include all narcotics, all antibiotics, antifungals, sedatives, sleeping pills, barbiturates, and all drugs in the Palliative Care Drug Plan (Plan P) formulary.
Prescriptions subject to the maximum 30-day supply for first fills policy include:
- A prescription for a new chemical entity
- A prescription that involves a change in dosage (strength or dosing frequency) of a previously prescribed chemical entity
- A new prescription for a previously prescribed chemical entity after a lengthy break in therapy (i.e., four months or longer)
PharmaNet cannot distinguish between first fills and refills. Pharmacists are responsible for determining whether a prescription is a first fill for a long-term maintenance drug (maximum 30-day supply) or a repeat fill (maximum 100-day supply). Pharmacists should review the client medication history and ask the prescribing physician or the client to determine whether the prescription is a first fill or a repeat fill.
The PharmaCare Formulary Search can be used to determine if a particular drug is subject to a 30-day maximum supply. Note that a 35-day maximum supply shown in the Formulary Search indicates a drug is subject to a 30-day maximum supply. The 35-day maximum supply shown is applicable only to Plan B clients.
The following are automatically exempt from the 30-day maximum days' supply:
- Claims under PharmaCare Plan B are exempt to accommodate the 35-day monitored dosage system that may be used by pharmacies servicing long-term care facilities, and
- Prescriptions dispensed under the Trial Prescription Program (where a 14-day trial has been dispensed)
In the case of individuals residing in rural or remote areas for whom travel to the pharmacy is a significant barrier, the pharmacist can call the PharmaCare Help Desk to request an exemption..
In the case of certain chronic conditions, physicians may submit a HLTH 5328 - General Special Authority Request asking PharmaCare to exempt a patient from the 30-day maximum supply for a short-term drug. Approval may be granted for a 100-day supply.
Visit Special Authority Coverage for details on exceeding maximum days’ supply.
Procedures
PROCEDURES FOR PHARMACISTS
Processing a prescription for a remote/rural exemption
To process a rural or remote exemption:
- Ensure that the client qualifies.
- Call the PharmaCare Help Desk and request the exemption.
The Help Desk will enter the exemption as a one-day Special Authority. - Write “rural supply exemption” on the prescription for audit purposes.
Correcting a claim when the 30-day supply limit has been mistakenly applied
If a 30-day supply limit has been applied mistakenly, the pharmacist may reverse the transaction and dispense a 100-day supply. This action is not eligible for a Special Services Fee.
Section 5.2 – Refilling Prescriptions Too Soon Policy
General Policy Description
PharmaCare does not normally cover prescription refills when there is more than a 14-day supply remaining from a previous fill.
Policy Details
PharmaCare does not cover prescription refills when there is more than a 14-day supply remaining from a previous fill, except when there is a legitimate reason for supplying the medication early and an appropriate intervention code is entered.
This policy does not apply to:
- Plan B (Long-Term Care) claims, or
- Top-up refill claims eligible under the Travel Supply Policy
For each claim submitted (with the exception of claims under PharmaCare Plan B and reversed claims), PharmaNet automatically:
- Adjudicates the current claim to zero if it is being submitted more than 14 days before the expiry of the days' supply of the previous claim and returns the response code CL – Exceeds Good Faith Limit
- Identifies the expiry date of the days' supply for the previous claim
- Reviews the days' supply of any previous claim for the same client and medication submitted up to 100 days before the current date
When there is a legitimate reason for supplying the medication early, pharmacists can submit the claim using the intervention code UF – Patient Gave Adequate Explanation.
Pharmacists are required to document the use of the UF intervention code, and the reason for supplying the medication early, in a manner accessible for audit purposes. PharmaCare Audit regularly reviews claims accompanied by the UF intervention code to ensure its legitimate use. Inadequate explanation and/or documentation of the use of the UF code may result in PharmaCare seeking recovery of costs.
Note that the CL response code does not supersede the Drug Use Evaluation (DUE) D7 – Fill Too Soon response. Although the DUE response is usually returned on claims that generate the CL response code, DUE response is based on medical data and continues to provide the most reliable alerts to potential drug therapy or dispensing problems.
Section 5.3 – Refilling Prescriptions on the Same Day Policy
General Policy Description
PharmaCare does not cover multiple fills of the same prescription for the same client on the same day unless an appropriate intervention code is entered in PharmaNet.
Policy Description
Coverage policy
PharmaCare does not cover drug costs or dispensing fees for multiple fills for the same DIN/PIN at the same pharmacy on the same day for the same client, except in certain circumstances with the associated intervention code entered in PharmaNet.
Lost or broken prescriptions
A lost or broken prescription fill processed with the intervention code MR – Item Lost or Broken is eligible for coverage even when refilled the same day as the initial prescription fill.
PRN supply claims for non-Plan B clients
When dispensing a prescription for a regular compliance-packed dose plus an extra PRN (“take as needed”) supply of a drug for a client not covered under Plan B, pharmacists should dispense the PRN portion in separate compliance packaging.
To permit the correct adjudication of the same day/same client PRN supply, use the intervention code CD – Therapeutic Duplication when entering the claim for the separate PRN portion.
The same day fill of the PRN claim, as with any other claim, must be supported by the physician’s authorization, which must be retained in the pharmacy’s prescription file.
PRN supply claims for Plan B clients
Pharmacies dispensing same day PRN (“take as needed”) supply claims to Plan B clients may use the intervention code MY – LTC Prescription Split for Compliance.
Claims for the same drug on the same day for a client and their pet
When both a client and their pet need the same drug on the same day and the pet’s prescription is filled first, PharmaNet interprets the subsequent,client prescription as a duplicate and rejects it. Although the claim record is removed from PharmaNet, the medication history record for the drug is not. So, if the claim is then re-entered with an intervention code, two fills of the medication will show on the client’s medication history.
When a pharmacy needs to fill a prescription for the same drug on the same day for both a client and their pet, the pharmacy should fill the client’s prescription first to prevent the potential for duplicate fill inaccuracies in the client’s medication history.
If no prescription for that PHN and drug has been filled earlier in the day, submit the:
- Client's prescription first
- Pet's prescription second. Include the veterinarian's Practitioner ID, the Reference Code V9 and the intervention code UF – Patient Gave Adequate Explanation
- If the client’s prescription was filled earlier in the day, submit the claim for the pet’s prescription. Include the veterinarian’s Practitioner ID, the Reference Code V9 and the intervention code UF – Patient Gave Adequate Explanation
- If the pet’s prescription was filled earlier in the day submit the claim for the patient’s prescription. Include the intervention code UF – Patient Gave Adequate Explanation
- If you forget to enter the UF intervention code and the claim is rejected, contact the PharmaNet Data Quality Team to correct the problem before you re-submit the prescription claim with the UF code
Section 5.4 – Travel Supply Policy
General Policy Description
Under the Travel Supply Policy, a pharmacist can provide an early prescription refill for clients travelling outside of B.C. Refills under the Travel Supply Policy are limited to topping up the remaining prescription supply to the maximum days’ supply recognized by PharmaCare for the drug.
The Travel Supply Policy is an exception to the Refilling Prescriptions Too Soon Policy, under which PharmaCare does not cover prescription refills when the patient has more than a 14-day supply remaining from a previous fill.
Policy Details
Eligibility
Clients are eligible for an early top-up refill under the Travel Supply Policy once every six months (180 days).
Coverage limit
PharmaCare covers travel supplies only up to the recognized PharmaCare maximum days' supply for the drug (i.e., 30 days for a short-term drug and 100 days for long-term). For example, if a patient has 25 days’ supply remaining of a drug subject to a 100-day supply maximum, a travel supply refill would be limited to a 75-day supply.
Clients requesting and receiving more than the recognized PharmaCare maximum days’ supply will be responsible for the cost of the supply exceeding the PharmaCare maximum days’ supply. For clients covered by Fair PharmaCare, only the portion of the costs eligible for PharmaCare coverage will count towards the deductible and/or family maximum.
Required documentation and recordkeeping
Clients must sign the Travel Declaration form (PDF, 122 KB) on the date the travel supply is filled in order to receive coverage for a travel supply refill. A parent or guardian can complete the Travel Declaration form for a child’s prescription.
Pharmacies can download the Travel Declaration form (PDF, 122 KB) or order them from Health Insurance BC (HIBC).
One Travel Declaration form can document several travel supply claims if multiple prescriptions are filled on the same day. If travel supply claims are filled on different days for the same client, a new form must be used each day.
Entering travel supply claims in PharmaNet
Pharmacies are to process travel supply claims using the intervention code MV – Vacation Supply.
Special Services Fee
Travel supply claims are not eligible for a Special Services Fee.
Procedures
PROCEDURES FOR PHARMACISTS
Processing a travel supply claim
If a client asks for an early refill for travel outside B.C., and the refill would be for more than 14 days, inform them of the Travel Supply Policy.
If the client requests coverage under the Travel Supply Policy:
- Have the client complete and sign a Travel Declaration form (PDF, 122 KB)
- Submit the claim with the intervention code MV – Vacation Supply
- Retain the signed Travel Declaration form for audit purposes
Response code 65–Intervention/Exception Code Error
If you enter a claim using the MV – Vacation Supply intervention code and receive the response 65‑Intervention/Exception Code Error, the client has less than a 14-day supply. Enter the claim without the MV intervention code.
If a patient chooses not to have a claim entered as a travel supply
Enter the claim with the intervention code DE – Adjudicate to $0.00 as requested. The patient will be responsible for the cost. If they are on the Fair PharmaCare plan, the cost will not count towards their deductible and/or family maximum.
Intervention codes that supersede the MV – Vacation Supply intervention code
If the MV–Vacation Supply intervention code is entered along with any one of the following intervention codes, the claim adjudicates according to the non-MV intervention code:
- MP–Valid Claim–Value $1000.00 to $9999.99
- MR–Replacement, Item Lost or Broken
- MX–Long Term Care PRN Order
- MY–Long Term Care Prescription Split for Compliance
- UA–Consulted Prescriber & filled Rx As Written
- UF–Patient Gave Adequate Explanation & Filled As Written
- DE–Adjudicate to $0.00 as Requested
The Travel Supply Policy cannot be applied if a client is moving outside of B.C. PharmaCare no longer covers a person's medication as soon as they move outside of B.C.
Section 5.4 Tools and Resources
Section 5.5 – Correct Quantities Policy
General Policy Description
Pharmacies must use the correct dispensed quantity when entering a claim in PharmaNet for the claim to adjudicate accurately.
Policy Details
When entering a claim in PharmaNet, pharmacies should use the unit of measure for the dispensed quantity that matches the one recognized in PharmaNet. Using the correct unit of measure will ensure accurate adjudication of the claim.
Claims that adjudicate for an incorrect dispensed quantity are subject to recovery by PharmaCare.
Procedures
Pharmacies may refer to Correct quantities for PharmaCare claims for the quantities to enter for particular products. The BC PharmaCare Formulary Search also provides units of measure for products.
Pharmacies may also contact the PharmaNet Help Desk for help determining correct quantities for claims.
Pharmacies may suggest additions to this list by sending an email to pharma@gov.bc.ca.
Procedures for entering correct quantities are explained in Section 3.13—Correct Quantities.
Section 5.6 – Maximum Pricing Policy
General Policy Description
PharmaCare sets a maximum price it will reimburse for drugs under the Drug Price Regulation. This maximum price is applied during PharmaNet claims adjudication. The maximum pricing policy also applies to some non-drug products.
Policy Details
PharmaCare reimburses drugs and some non-drug products eligible for PharmaCare coverage up to a maximum price based on:
- The sum of the manufacturer list price and 8% of that price for most drugs
- The sum of the manufacturer list price and 5% or less for drugs subject to the High-Cost Drugs Policy
The level of PharmaCare reimbursement may be further limited when the product is subject to the Reference Drug Program (RDP), the Low Cost Alternative program, a price established in an agreement entered into under the Pharmaceutical Services Act, or another PharmaCare pricing program or policy.
PharmaCare reimburses the lesser of the costs adjudicated under all pricing programs and policies applicable to a particular product.
Procedures
When entering a claim in PharmaNet, use the correct unit of measure as per the Correct Quantities Policy. PharmaNet divides the product cost by the dispensed quantity and compares the result to the PharmaCare pricing rules for the product.
Refer to the Correct Quantities list or the BC PharmaCare Formulary Search to check the unit of measure used for a product’s PharmaCare pricing.
Section 5.7 – Actual Acquisition Cost Policy
General Policy Description
For certain products, PharmaCare covers only the pharmacy’s actual cost of procuring the product.
Policy Details
For products subject to the Actual Acquisition Cost (AAC) policy, PharmaCare reimbursement will not exceed the provider’s AAC for the product up to a maximum price based on the manufacturer list price plus a 7% mark-up.
The product cost submitted in PharmaNet is to be reduced by any volume rebates or free goods received. Actual freight costs can be included in the AAC.
A discount paid or credited by a supplier for prompt payment of invoices is not included in the calculation of AAC. (The PharmaCare-recognized discount is usually no more than 2%.)
When submitting claims, the Drug Cost field should contain only the AAC. Charges in excess of the AAC must be entered in the Drug Upcharge field; these are not part of the claim value that is adjudicated.
PharmaCare will recover overpayments made as a result of claims submitted above the AAC.
Product costs submitted in excess of a PharmaCare maximum price will be adjudicated based on the applicable maximum price and any other applicable PharmaCare pricing policies.
Products subject to this policy, reimbursed at AAC up to the PharmaCare maximum price plus a dispensing fee (up to the PharmaCare maximum dispensing fee), include:
- Blood glucose test strips and continuous/flash glucose monitors
- Cystic fibrosis vitamin and nutritional supplements
Procedures
Entering claims under the AAC policy
Enter the AAC of the product in the PharmaNet Drug Cost field.
Enter any product cost in excess of the AAC in the Drug Upcharge field, not the Drug Cost field.
When entering a claim in PharmaNet, use the correct unit of measure. Refer to the Correct Quantities list or the BC PharmaCare Formulary Search.
Section 5.8 – High-Cost Drugs Policy
General Policy Description
Certain high-cost drugs are subject to specific reimbursement limits under the Drug Price Regulation.
Policy Details
PharmaCare reimburses drugs designated as high-cost drugs under the Drug Price Regulation up to a maximum price based on the sum of the manufacturer list price for the drug and specific percentage of that price (usually 5% or less).
PharmaCare considers the total cost of the drug per patient, which is partially dependent on the expected duration of use, when determining whether a drug should be designated a high-cost drug.
Refer to the List of Designated High-Cost Drugs.
Procedures
When entering a claim in PharmaNet, use the unit of measure. Refer to the Correct Quantities list or the BC PharmaCare Formulary Search.
Section 5.8 Tools and Resources
Section 5.9 – Retail Pricing Policy
General Policy Description
For certain products, PharmaCare covers the retail price of the product.
Policy Details
For products subject to the Retail Pricing Policy, PharmaCare reimburses the retail price of the product with no dispensing fee.
Products subject to this policy include the following:
- Reimbursed up to a maximum of the manufacturer's suggested retail price for the product with no dispensing fee:
- Reimbursed at the regular retail price with no dispensing fee:
- Insulin - regular, long-acting, short-acting, rapid-acting insulin analogues
- Needles/syringes for insulin therapy
- Ostomy supplies
- Reimbursed at retail price up to the maximum retail price set by the First Nations Health Authority (FNHA) with no dispensing fee:
Section 5.10 – Full Payment Policy
General Policy Description
Under the Full Payment Policy, pharmacies are not permitted to charge any amount directly to clients who are receiving full PharmaCare coverage for a claim.
Policy Details
When the Full Payment Policy applies
The Full Payment Policy applies to all pharmacy providers that submit claims to PharmaCare.
Pharmacies must not charge any amount directly to an individual receiving full PharmaCare coverage for a drug, substance, or related service that is a full benefit.
Individuals receiving full PharmaCare coverage are those covered under PharmaCare plans B, C, D, F, G, M, P, W, Z, and those that have reached their Fair PharmaCare family maximum.
A drug, substance, or related service is a full benefit when it is:
- A regular PharmaCare benefit (that is, no Special Authority required)
- A limited coverage drug for which Special Authority approval has been granted
- Not subject to any reimbursement limit on adjudication under the Low Cost Alternative program or Reference Drug Program
When the Full Payment Policy does not apply
The Full Payment Policy does not apply when the drug, substance, or related service:
- Is not a PharmaCare benefit
- Adjudicates as a partial PharmaCare benefit under the Low Cost Alternative program or Reference Drug Program
- Is exempt from the Full Payment Policy
The following products are exempt from the Full Payment Policy:
- Insulin
- Medical supplies and devices, including
- prosthetics
- orthotics
- ostomy supplies
- cystic fibrosis nutritional supplements/vitamins
- diabetes supplies (insulin pumps and supplies, needles and syringes, continuous/flash glucose monitors and blood glucose test strips)
This exemption does not include copper IUDs.
Charges in error
When a pharmacy provider mistakenly charges drug, substance, related service, or dispensing fee costs directly to an individual for a claim the Full Payment policy applies to, the pharmacy provider must refund those charges.
Procedures
When submitting claims that the Full Payment Policy applies to, do not charge clients for any amount in excess of that accepted for reimbursement by PharmaCare
Policy in Practice
For a client covered under several plans, the Full Payment Policy might not apply to all of their prescriptions. For example, Roberto presents two prescriptions, one for a psychiatric medication covered under Plan G and the other for an ointment covered under Fair PharmaCare. The claim for the psychiatric medication will be subject to the Full Payment Policy. Robert has met his Fair PharmaCare deductible but has not yet reached his family maximum, so the claim for the ointment will not be subject to the Full Payment Policy.
If a pharmacy provider is able to determine that the client’s private insurer will cover any costs in excess of the amount PharmaCare covers, they can charge the private insurer. The determination of coverage by the private insurer must be made at the time the product is dispensed. A pharmacy provider cannot charge a patient on the understanding that a private insurer may pay all or some of the cost at a later date.
Section 5.11 – Low Cost Alternative Program
General Policy Description
The Low Cost Alternative (LCA) program, established under the Drug Price Regulation, is intended to ensure the best value is obtained for expenditures on multi-source drugs. The program limits PharmaCare reimbursements for drugs subject to an LCA price.
Drugs in the LCA program may be subject to the Reference Drug Program (RDP). Unlike the LCA program, the RDP applies to drugs with different active ingredients that are in the same therapeutic category and treat the same conditions.
Policy Details
General LCA program policies
The LCA program groups drugs into categories of products with the same active ingredient or combination of active ingredients, and the same strength.
Each category is assigned an LCA price.
The maximum PharmaCare reimburses for drugs within the same LCA category is the lesser of the:
- LCA price
- RDP price, if the drug is also subject to the RDP
Benefit status
Under the LCA program, generic drugs are reimbursed as full benefits.
Brand drugs are covered as partial benefits, unless their list price is equal to, or less than, the LCA price for the category, in which case they are reimbursed as full benefits (some exceptions apply).
For information about LCA drugs, categories, prices, and benefit status, refer to the Low Cost Alternative (LCA) and Reference Drug Program (RDP) Data Files.
Pharmacists may also call the PharmaCare Help Desk for information.
LCA price for a category
The LCA price includes an 8% mark-up or, for drugs that are subject to the High-Cost Drugs Policy, the maximum mark-up indicated in the List of Designated High-Cost Drugs.
The LCA price may be based on a pre-determined maximum accepted list price (MALP), may be granted an exception to the MALP, or may be based on pricing resulting from the pan-Canadian Pharmaceutical Alliance (pCPA) generic initiative, effective April 1, 2018.
Submission types
As described in the following sections, under the LCA program, PharmaCare accepts two types of drug submissions from manufacturers:
- Regular submissions, in which a drug will be subject to the MALP for the category unless it qualifies for an exception to the MALP or for provisional listing
- Submissions for a drug included in the pCPA generic initiative, under which the generic drug is subject to a price limit
Regular submissions: drugs subject to the maximum accepted list price (MALP)
For each LCA category, PharmaCare determines a MALP for generic drugs within the category.
Calculation of the MALP differs depending on when an LCA category was established.
PharmaCare may grant an exception to the MALP under certain circumstances.
MALP for LCA categories established on or after April 1, 2013
LCA comparators
For LCA categories established on or after April 1, 2013, an LCA comparator is designated and used to determine the MALP for generic drugs in each LCA category.
The designated LCA drug comparator is the:
- Brand drug in the LCA category when there is a single brand drug in the category
- Most cost-effective brand drug in the LCA category when there is more than one brand drug in the category
- LCA drug comparator of another LCA category consisting of drugs with the same active ingredient or combination of active ingredients but a different strength, when there is no brand drug in a category
- LCA drug comparator used by another jurisdiction (e.g., Ontario or other) if the addition or removal of a generic drug causes the LCA category to shift tiers in accordance to the Tiered Pricing Framework
MALP calculation
Under Section 4 of the Drug Price Regulation, the MALP is calculated as a percentage of the manufacturer list price for the LCA comparator for that category.
Effective April 1, 2019, MALPs for LCA categories established on or after April 1, 2013, are calculated as follows:
- 25% of the LCA comparator list price for LCA categories consisting of drugs available as oral solids only
- 35% of the LCA comparator list price for any other LCA category
If an LCA category contains no brand drugs, the MALP for that LCA category is calculated as a proportion, by strength, of the MALP for the LCA category to which the designated LCA comparator belongs.
Refer to 2019 Amendments to B.C. Drug Price Regulation (PDF, 268KB).
MALP for LCA categories established before April 1, 2013
LCA comparators
For LCA categories established before April 1, 2013, a base price has been established in the Schedule to the Drug Price Regulation.
MALP calculation
Under Section 5 of the Drug Price Regulation, the MALP is calculated as a percentage of the base price for each LCA category.
Effective April 1, 2019, MALPs for LCA categories established before April 1, 2013, are calculated as follows:
- 25% of the brand price for LCA categories consisting of drugs available as oral solids only
- 35% of the brand price for any other LCA category
If a drug in an LCA category had, as of July 1, 2010, a lower manufacturer list price than the calculated MALP for the LCA category to which it belongs, the MALP for that LCA category will be the manufacturer list price of that drug as of July 1, 2010.
MALP exceptions
In certain circumstances, PharmaCare may increase the effective MALP for a category from the one initially calculated (e.g., where proper documentation indicates that a manufacturer’s cost to produce and distribute a generic product is greater than the MALP).
PharmaCare will not consider a request for an exception if the drug is being supplied or is intended to be supplied in another jurisdiction in Canada at a list price that is equal to or lower than the MALP as initially calculated by PharmaCare, or at a list price higher than the list price submitted in another jurisdiction.
If no manufacturer commits to sell a generic drug at or below the calculated MALP for an LCA category, or if PharmaCare has significant concerns with the sufficiency of supply offered by the manufacturers committing to sell at or below the MALP, PharmaCare will review the exception requests and prices submitted by manufacturers for generic drugs in the category. Based on its review, PharmaCare may, at its sole discretion, take whatever action it deems appropriate, which may include, without limitation, one or more of the following:
- PharmaCare may grant an exception to the MALP requirement to the manufacturer(s) submitting the lowest list price, or a lower list price for any drug in the LCA category
- Where necessary to ensure sufficient supply for PharmaCare beneficiaries, PharmaCare may grant exceptions to more than one drug in an LCA category
- In such instances, drugs will be considered for coverage in order of submitted list price, with the lowest priced drug receiving first consideration
- Such exceptions are granted on a provisional basis, meaning that the exception may be rescinded at any time after PharmaCare has deemed a product to be accepted. If an exception is rescinded, manufacturers will have an opportunity to resubmit the product at any time
- PharmaCare may deem all generic drugs in the LCA category ineligible for PharmaCare coverage
- PharmaCare may deem all drugs in the LCA category ineligible for PharmaCare coverage
- PharmaCare may undertake a competitive process to ascertain the lowest price at which a manufacturer is prepared to supply the drug in question for PharmaCare beneficiaries
Impact of the MALP on eligibility for PharmaCare coverage
A generic drug that is, or would be, assigned to an LCA category and that has a manufacturer list price exceeding the applicable MALP is not eligible for PharmaCare coverage, unless an exception to MALP is granted on a provisional basis.
If a generic drug is deemed ineligible for PharmaCare coverage because the manufacturer list price exceeds the MALP, the manufacturer may resubmit the drug for reconsideration at any time. Temporary coverage may be provided to certain drugs under Section 16 of the Drug Price Regulation.
Drugs subject to the LCA Program that are determined to be ineligible for PharmaCare coverage are published in the “Non-Benefits” worksheet in the Low Cost Alternative (LCA) and Reference Drug Program (RDP) Data Files.
Submissions for drugs included in the pan-Canadian Pharmaceutical Alliance generic initiative
On January 29, 2018, the pan-Canadian Pharmaceutical Alliance (pCPA) and the Canadian Generic Pharmaceutical Association (CGPA) announced a five-year initiative with participating provinces and territories, including British Columbia. Under this initiative, the prices of nearly 70 of the most commonly prescribed generic drugs were reduced effective April 1, 2018.
See specific pricing for drugs included in the initiative.
PharmaCare uses the LCA Program to implement pricing under this initiative.
PharmaCare does not cover a generic product subject to this initiative if its price exceeds that established under the initiative.
Products and pricing under this initiative are identified in the Low Cost Alternative (LCA) and Reference Drug Program (RDP) Data Files.
LCA program and RDP data files
The PharmaCare website provides regularly updated LCA program and RDP drug information in downloadable Excel spreadsheets updated on the first Thursday of every month. Visit Low Cost Alternative (LCA) and Reference Drug Program (RDP) Data Files
PharmaCare typically provides at least 14 days prior notice of any changes to categories, included products, Tiered Pricing Framework price adjustments, reimbursement limits and product benefit status in the LCA/RDP Updates Workbook
On occasion, a change to the LCA program or RDP may come into effect between the regular monthly updates, including but not limited to the
- Implementation of generic drug pricing adjustments
- Expedited implementation of coverage for significant first-entry generic drugs
If a change comes into effect between the regular monthly updates, PharmaCare publishes updated versions of the LCA and RDP spreadsheets and the LCA/RDP Updates Workbook online. Check the website on the first Thursday of the month and then again in the middle of the month to ensure you always have the most recent LCA/RDP information.
Interchangeability
The LCA and RDP spreadsheets should not be considered an endorsement by PharmaCare of the interchangeability of any products identified. The College of Pharmacists of B.C. has delegated the responsibility for determining the interchangeability of products to B.C. pharmacists. Pharmacists are advised to consult with the prescriber to obtain the appropriate legal authority to dispense an LCA or RDP product.
Client impact
If a client’s prescription is for a partial benefit product, the pharmacy is encouraged to make the client aware that there is a full benefit LCA or RDP product that provides the same or similar therapeutic treatment.
If the client chooses the partial benefit product, they will be required to pay any cost in excess of the LCA or RDP price.
When being dispensed a drug subject to the LCA program, clients have three choices:
- Obtain a full benefit product which will be fully reimbursed up to the PharmaCare maximum price (or RDP price, where applicable)
- Obtain a partial benefit product and pay any portion of the drug cost that exceeds the LCA price (or RDP price, where applicable). Fair PharmaCare clients should be made aware that only the portion of the drug cost accepted for reimbursement by PharmaCare counts toward their deductible
- Have their physician request Special Authority approval for full benefit coverage of a partial benefit product where individual circumstances warrant
Special Authority approval for LCA drugs
If a client has an allergy or intolerance to the non-therapeutic ingredients in the majority of generic products in an LCA category, a physician may apply to PharmaCare for Special Authority approval for full benefit coverage of a brand name product in that category.
When a Special Authority is granted, coverage is provided up to the PharmaCare maximum price for the drug, as reflected in the Maximum Pricing Policy.
A Special Authority does not override plan deductibles or co-payments.
Learn more at Special Authority
Manufacturer shortages
Shortages of a particular full benefit product in an LCA category may be addressed by stocking a different full benefit product within the same category for the duration of the shortage.
If a shortage impacts all of the full benefit products within an LCA category, PharmaCare will verify the shortage with the manufacturers and determine the expected duration. When such a shortage may impact the availability of a sufficient supply of full benefit products, PharmaCare will implement measures to ensure supply needs are met. These measures will be communicated to pharmacies and may include:
- Temporary removal of the Special Authority requirement for full coverage of partial benefit product(s) in an LCA category
- Temporary PharmaCare coverage of a product or products not currently listed for coverage in an LCA category
It is recommended that pharmacies confirm the anticipated duration of a shortage before ordering an inventory of a drug provided a temporary change in benefit status. When a shortage is over, PharmaCare will rescind temporary changes in benefit status with minimal notice.
Pharmacies should contact the PharmaCare Help Desk to report shortages or inquire about measures being taken to address a shortage.
Section 5.11 Tools and Resources
Section 5.12 – Reference Drug Program
General Policy Description
The PharmaCare Reference Drug Program (RDP), established under the Drug Price Regulation, encourages cost-effective, first-line prescribing for common medical conditions by limiting reimbursement for certain drugs in designated therapeutic categories to a maximum daily amount payable.
Whereas the Low Cost Alternative Program applies to drugs that have identical active ingredients, the Reference Drug Program applies to drugs that are not identical but are part of the same therapeutic category and are used to treat the same conditions.
Drugs included in the Reference Drug Program may also be subject to the Low Cost Alternative Program.
Policy Details
GENERAL REFERENCE DRUG PROGRAM POLICIES:
Effective December 1, 2016, the Reference Drug Program currently applies to eight categories of drugs:
- Angiotensin-converting enzyme inhibitors (ACE inhibitors)
- Angiotensin receptor blockers (ARBs)
- Dihydropyridine calcium channel blockers (CCBs)
- HMGT-CoA reductase inhibitors (statins)
- Histamine2 receptor blockers (H2 blockers)
- Nitrates
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Proton pump inhibitors (PPIs)
Designated reference drugs in each category are reimbursed up to the lesser of any applicable Low Cost Alternative Program price and the drug’s PharmaCare maximum price as reflected in the Maximum Pricing Policy.
For each category, one drug is designated as the reference drug comparator and is used to determine the maximum daily amount payable for non-reference drugs in the category.
The maximum daily amount payable for each category changes in relation to any change in the reference drug comparator itself or to any change in the price of the reference drug comparator, including changes resulting from Low Cost Alternative Program pricing.
PharmaCare limits reimbursement for non-reference drugs in each category to the applicable maximum daily amount payable.
Low Cost Alternative and Reference Drug Program data files
PharmaCare publishes the following information online in the Low Cost Alternative (LCA) and Reference Drug Program (RDP) data files:
- Reference drugs
- Non-reference drugs
- Maximum daily amounts payable for non-reference drugs
- Reference drug comparators
- Daily therapeutic doses used to determine maximum daily amounts payable
You can also consult the list of full and partial RDP benefits.
Interchangeability
The PharmaCare Low Cost Alternative (LCA) and Reference Drug Program (RDP) Data Files should not be considered an endorsement by PharmaCare of the interchangeability of any products identified.
The College of Pharmacists of B.C. has delegated the responsibility for determining the interchangeability of products to B.C. pharmacists.
Pharmacists are advised to consult with the prescriber to obtain the appropriate legal authority to dispense an LCA or RDP product.
Patient impact
If a patient’s prescription is for a non-reference drug, the pharmacy is encouraged to make the patient aware of the fully covered reference drug(s)and that the reference drug(s) provide the same or similar therapeutic treatment.
If the patient chooses the non-reference drug, they will be required to pay any amount in excess of the applicable maximum daily amount payable (and LCA price, where applicable).
PharmaCare beneficiaries taking a non-reference drug have three options:
- Switch to a reference drug
- Remain on the non-reference drug and pay any cost in excess of the amount reimbursable under the Reference Drug Program (or Low Cost Alternative Program, where applicable)
Fair PharmaCare patients should be made aware that only the lower amount (i.e., the amount reimbursable under the Reference Drug Program) will accumulate toward the family deductible, even if they choose to pay the price difference for the non-reference drug - Have their physician request a Special Authority for full reimbursement of a non-reference drug, when individual circumstances warrant
Special Authorities for Reference Drug Program drugs
The Special Authority process is designed to ensure access to drugs based on patient-specific needs.
If a patient has a specific medical condition that prevents them from taking the reference drug(s) in an RDP category (e.g., a drug-to-drug interaction, drug intolerance or previous treatment failure), their physician may submit a Special Authority Request for full benefit coverage of a non-reference drug.
When a Special Authority is granted, coverage will be provided up to the lesser of:
- Any applicable Low Cost Alternative price for the product, or
- The claimed drug cost or the maximum allowable price for the drug, as defined by the Maximum Pricing Policy
A Special Authority does not override plan deductibles or co-payments.
>> See Special Authority process and criteria for specific products.
Procedures
Claims for drugs subject to the Reference Drug Program can be claimed in the normal manner. PharmaNet automatically adjudicates the claim under the Reference Drug Program and any other applicable pricing program or policy.
Section 5.12 Tools and Resources
Section 5.13 – Compounded Prescriptions
General Policy Description
Pharmacare recognizes compounded prescriptions as rational combinations of active ingredients requiring professional judgment and technical skill in their preparation.
PharmaCare reimburses pharmacies for specific compounds and for compounding costs, up to certain limits.
Policy Details
Compounds eligible for coverage
- No suitable alternative is available commercially (e.g., different brand, different drug, etc.), and
- The specific eligibility criteria for the ingredient (as noted below) are met, and
- The pharmacy has a current medical practitioner's prescription for the compound on file, and
- The compound is produced by trained staff with appropriate expertise using appropriate and cost-effective ingredients and procedures
PharmaCare covers other types of compounds (i.e., not on the list below) only if Special Authority approval is in place before the compound is dispensed.
If you are unsure if PharmaCare will cover a specific compound, contact the PharmaCare Help Desk before submitting the claim in PharmaNet.
ELIGIBLE COMPOUNDS
| Eligible compound | Criteria for coverage |
|---|---|
| Oral solutions | N/A |
| Oral suspensions | Oral suspensions are a benefit only:
Pharmacists must document on the original prescription the reason the client requires a suspension. |
| Dermatological compounds | Dermatological compounds are a benefit when all of the following conditions are met:
|
| Topical antifungals | Topical antifungal compounds are a benefit only if the client has Special Authority approval for the specific active ingredient |
| Retinoic acid | Retinoic acid compounds are a benefit only if the client has Special Authority approval for the specific active ingredient
|
| Preservative-free sterile eye drops | Preservative-free sterile eye drops are a benefit when:
|
| Plan P injectable analgesics: continuous ambulatory delivery device (CADD) | The required repackaging of a prescribed injectable Plan P benefit medication(s) into a CADD pump is a compound benefit when the client is registered for Plan P |
| Plan P intrathecal analgesics | Intrathecal analgesic compounds are a benefit for clients registered for Plan P |
DERMATOLOGICAL COMPOUNDS—ELIGIBLE ACTIVE INGREDIENTS AND CRITERIA FOR COVERAGE
| Eligible active ingredient | Coverage criteria |
|---|---|
| Anthralin | For psoriasis, eczema, and other severe dermatological conditions |
| Camphor | Only when in combination with at least one prescription benefit ingredient |
| Clindamycin in Duonalc™ | Only clindamycin in Duonalc™ is a benefit; clindamycin in Reversa™ or any other over‑the-counter medicated base is not a benefit |
| Coal tar | None |
| Corticosteroids | Only when used as additives or as a medicated benefit base (e.g., hydrocortisone, betamethasone, clobetasol). Preparations containing a corticosteroid compounded with a non-benefit or over‑the‑counter medicated product are not benefits. |
| Erythromycin | None |
| Liquor carbonis detergens (LCD) | None |
| Menthol | Only in combination with at least one prescription benefit ingredient |
| Metronidazole | None |
| Salicylic acid | For psoriasis, eczema, and other severe dermatological conditions |
| Sulfur, sulfacetamide | When added to, or combined with, a medicated benefit base |
DERMATOLOGICAL COMPOUNDS—ELIGIBLE NON-MEDICATED BASES
If a non-medicated base is not listed below, contact the PharmCare Help Desk before submitting a claim.
- Aquaphor™
- Aquatain™
- Cetaphil®
- Cliniderm™
- Cold cream
- Complex 15™
- Dermabase™
- Dilusol™
- Dormer™
- Duonalc™
- Emollient cream
- Eucerin™
- Glaxal™
- Hydrophilic petrolatum +/- 25% water
- Lanolin
- Lubriderm®
- Medi-Derm™
- Moisturel™
- Neutrogena®
- Nutraderm™
- Spectro Gel™
- Unibase®
- Vanishing cream
- Vaseline™
INELIGIBLE COMPOUNDS
PharmaCare does not cover compounds containing non-benefit ingredients. If a compound is not eligible for PharmaCare coverage but is claimed using a PIN for a benefit compound, the claim is subject to recovery.
DISCONTINUED PRODUCTS
PharmaCare does not cover compounds intended to replace commercially available products that have been discontinued by the manufacturer, even if the commercial product was a benefit.
Special Authority coverage for compounds to replace discontinued products may be requested only after the client’s practitioner has:
- Reassessed the client’s need for that specific drug and dosage form, and
- Contacted Health Canada’s Special Access Program to determine if the product is available through that route
MANUFACTURER SHORTAGE
PharmaCare does not automatically cover compounds intended to replace products unavailable due to a manufacturer shortage. When notified of a shortage, PharmaCare will first verify the shortage and expected duration of the shortage with the manufacturer.
If a shortage is expected to last for an extended period of time, PharmaCare normally establishes a specific PIN and maximum price in PharmaNet for replacement compounds. Replacement compounds can then be claimed using these PINs for the duration of the shortage. The submission of Special Authority requests is not required if a specific PIN has been assigned. The PIN assigned must be in place before a claim is submitted.
If PharmaCare has not assigned a PIN for a replacement compound, Special Authority approval is required.
Refer to Drug shortages for information about current drug shortages and substitutions.
SPECIAL AUTHORITY FOR NON-BENEFIT COMPOUNDS
PharmaCare recognizes that there may be exceptional, last-resort circumstances that justify coverage of non‑benefit compounds. Special Authority approval is required for PharmaCare coverage of non-benefit compound prescriptions.
Such requests are reviewed on a case-by-case basis. PharmaCare may approve full, partial or no coverage. If Special Authority coverage for a compound is approved, PharmaCare provides the pharmacy with a specific PIN that must be used when submitting the claim.
Pharmacies are required to submit a HLTH 5425 - Compound Costing Worksheet (PDF, 521KB) for compounds that require Special Authority approval. A copy of the approved Compound Costing Worksheet must be retained on file with the original prescription.
Refer to Submitting a Special Authority request for a compound
MAXIMUM ALLOWABLE FEES
The maximum allowable compounding fees that PharmaCare will reimburse are specified in the table below. If a compound does not appear in the table below, PharmaCare Special Authority will review compounding costs on a case-by-case basis and determine an appropriate compounding fee.
Compounding fees must be added to the ingredient costs and the combined amount entered in the Drug Cost field in PharmaNet. Do not include the compounding fee in the Dispensing Fee field.
Compounding fees in excess of PharmaCare maximums—or the amount approved by Special Authority—must not be claimed in the Drug Cost field. Upcharges on compounding fees must be entered in the Cost Upcharge field so that the amount becomes payable by the client or their alternate insurer.
The table below lists maximum fees for both benefit compounds and compounds approved by Special Authority that would otherwise be a non-benefit. The inclusion of a fee for a particular type of compound, therefore, does not necessarily mean benefit status.
| Compound | Maximum allowable compound fee |
| Oral solutions | $20.00 |
| Oral suspensions | $20.00 |
| Capsules | $0.30 per capsule |
| Suppositories | $40.00 * |
| Oral lozenges | $40.00 * |
| CADD injections | $20.00 |
| Sterile IV, IM, SC injections | $20.00 |
| Intrathecal injections | $40.00 |
| Creams/ointments/lotions < or = 250 gm/mL | $15.00 |
| Creams/ointments/lotions > or = 251 gm/mL | $20.00 |
| Sterile eye drops, preservative-free | $30.00 |
* Where appropriate (e.g., when the prescription will be dispensed on a frequent, short days’ supply basis), the compounding fee for suppositories and oral lozenges will be pro-rated during Special Authority adjudication.
RELATIONSHIP OF COMPOUNDING FEES TO DISPENSING FEES
Pharmacies can claim both their usual dispensing fee and a compounding fee.
INGREDIENT COSTS
Ingredient costs for compounds are subject to all PharmaCare Pricing Policies.
Ingredient costs for commercial products must be claimed at the lowest of any pricing policy applicable to the product (e.g., the Maximum Pricing Policy, Low Cost Alternative Program, Reference Drug Program).
Raw ingredients are subject to the Actual Acquisition Cost Policy.
PharmaCare expects pharmacies to use the most reasonably priced ingredients when compounding PharmaCare benefits. For example:
- Omeprazole—use powder instead of capsules
- Sodium bicarbonate—use powder and water instead sodium bicarbonate injectable for oral/topical products that do not require sterility
EQUIPMENT AND SUPPLY COSTS
PharmaCare covers the cost of the following supplies and equipment:
- Required supplies and special packaging such as gelatin capsules, cassettes/bags/syringes for administration devices, IV bags, adapta-caps, EMP jars
- Disposable, required supplies and equipment applicable to a particular compound claim, such as weighing boats, syringes, filters, needles, for compounding/measuring.
Reimbursement for eligible supplies and equipment is subject to the Actual Acquisition Cost Policy.
PharmaCare does not cover these costs:
- Charges for equipment use, lab fees, pH metre fees, etc.
- Cost of gowns, booties, and similar items
PRODUCT IDENTIFICATION NUMBERS (PINs)
Enter claims for prescription compounds using the applicable benefit or non-benefit compound PIN.
Claims for non-benefit compounds that are submitted using a PIN for a benefit compound are subject to recovery.
When Special Authority coverage for a compound is approved, PharmaCare will provide the pharmacy with a specific PIN for use when submitting the claim. Pharmacists must use that specific PIN when submitting the claim to PharmaNet.
Refer to Compounded Prescriptions PINs for the list of PINs.
CONTRACTED COMPOUNDING SERVICES
When a pharmacy contracts another pharmacy to provide a compound for individual prescriptions, the dispensing pharmacy may not claim more than is permitted under the PharmaCare Compounded Prescriptions policy.
When determining if contracting this service is appropriate, the dispensing pharmacy should ensure the contracted pharmacy will provide the compound at a cost within PharmaCare policy limits.
If a pharmacy contracts another pharmacy to provide a compound, the pharmacy must ask the compounding pharmacy for a cost breakdown and must retain that cost breakdown on file in keeping with the recordkeeping requirements below.
For benefit compounds, pharmacies must document the following information and retain it on file with the original prescription:
- Compound ingredients and their concentration, dosage form, and quantity
- Itemized cost of each ingredient and total ingredient costs
- Itemized supply and equipment costs and total costs
- Compounding fee
This information may be recorded on the prescription or on a separate document attached to the prescription. The HLTH 5425 - Compound Costing Worksheet (PDF, 521KB) may be used for this purpose.
If a change in costs occurs for a refill of a compound, pharmacies must complete and retain a new compound costing document, including all the information above.
For compounds requiring Special Authority, pharmacies must retain on file with the original prescription a copy of the SA-approved HLTH 5425 - Compound Costing Worksheet (PDF, 521KB).
Procedures for pharmacists
SUBMITTING A CLAIM FOR A COMPOUND IN PHARMANET
When submitting a claim for a compound:
- In the Drug Cost field, enter the combined amount for:
- eligible ingredient costs, plus
- eligible compounding fee, plus
- eligible equipment and supply costs
Do not add the compounding fee to the Dispensing Fee field.
- In the Dispensing Fee field, enter your usual dispensing fee
- In the Upcharge field, enter any portion of the compounding fee, ingredient costs, or supply/equipment costs that exceeds PharmaCare reimbursement maximums or the amounts approved by Special Authority. These amounts will then be payable by the client or their alternate insurer
- Enter the relevant PIN from the Compounded Prescription PINs or the specific Special Authority PIN.
- To ensure complete information is available to other healthcare providers using PharmaNet, enter the following at the beginning of the Directions for Use (SIG) field:
- If PIN identifies the active ingredient(s): Enter the dose and/or concentration along with the directions for use
- If PIN does not adequately identify the active ingredient: Enter the active ingredient(s), the dose and/or concentration, along with the directions for use
|
BENEFIT COMPOUND SUSPENSIONS |
|
|---|---|
|
Benefit compound PINs are drug/dosage form specific. To ensure the dose and concentration is available to other PharmaNet users, enter it exactly as indicated in the example below: |
|
|
Prescription |
felodipine 10 mg (in suspension) once daily |
|
PIN |
22123241 |
|
Description in PharmaNet |
felodipine compounded suspension |
|
Directions for SIG field |
Take 5 mL (10 mg) once daily |
|
PALLIATIVE BENEFIT CADD PUMP COMPOUNDS |
|
|
CADD pump PINs specify whether or not the ingredient is narcotic. The dose is usually included in the directions of the prescription. To identify the specific drug being used, enter it exactly as indicated in the example below. |
|
|
Prescription |
hydromorphone 20 mg/mL for CADD. 3.5 mg/hr and 7 mg BTP q20min |
|
PIN |
22123288 |
|
Description in PharmaNet |
narcotic CADD pump compound: palliative |
|
Directions for SIG field |
Run 3.5 mg/hr (hydromorphone) and 7 mg for BTP every 20 min |
|
BENEFIT DERMATOLOGICAL COMPOUNDS |
|
|
PINs for these medications identify the ingredient or class of ingredients. Pharmacists are not required to enter the specific corticosteroid or concentration of ingredients. Example below. |
|
|
Prescription |
HC 1% + menthol 0.25% cream. BID |
|
PIN |
22123278 |
|
Description in PharmaNet |
corticosteroid + menthol +/or camphor |
|
Directions for SIG field |
Enter usual directions for use. |
|
BENEFIT COMPOUNDED PRESERVATIVE-FREE EYEDROPS |
|
|---|---|
|
PINS for eyedrops identify the active ingredient and that the eyedrops are preservative-free. Add the concentration of the eyedrops to the directions as indicated in the example below. |
|
|
Prescription |
timolol 0.25% preservative-free eyedrops. 1 gtt BID |
|
PIN |
22123295 |
|
Description in PharmaNet |
timolol PF cpd eyedrop |
|
Directions for SIG field |
Instil 1 drop (0.25%) in left eye twice daily. |
|
PALLIATIVE BENEFIT COMPOUNDED INTRATHECAL INJECTIONS |
|
|
Due to wide variation in ingredients and concentration of ingredients, the PINs give only a general description. It is not necessary to add information to the usual instructions for use. Example below. |
|
|
Prescription |
intrathecal fentanyl 1000 mcg + bupivicaine 40 mcg + clonidine 100 mcg/mL |
|
PIN |
22123302 |
|
Description in PharmaNet |
narcotic + non-narcotic intrathecal cpd: palliative |
|
Directions for SIG field |
Enter usual directions for use. |
Retain the original prescription and supporting documentation on file (refer to Recordkeeping Requirements).
SUBMITTING A SPECIAL AUTHORITY REQUEST FOR A COMPOUND
To obtain Special Authority approval, the following documentation is needed.
From a medical practitioner:
- A Compound Coverage Request (PDF, 536KB) indicating:
- the compound prescribed
- why the compound is required for the particular client
- the name of the compounding pharmacy (so that PharmaCare can contact the pharmacy)
For certain palliative care compounds, a copy of the prescription may suffice as supporting documentation. Contact the PharmaCare Help Desk to determine the documents required..
From a pharmacy:
- A HLTH 5425 - Compound Costing Worksheet (PDF, 521KB) including:
- drug, concentration, dosage form, and quantity
- itemized cost of each ingredient
- itemized supply and equipment costs
- days’ supply
- time required to compound (active compounding time only; do not include set‑up, cleaning, or administrative time)
- Retain a copy of the Compound Costing Worksheet on file with the prescription.
- Wait for PharmaCare Special Authority approval before dispensing the compound
When PharmaCare receives the Special Authority Request and cost breakdown, it will decide the amount, if any, that is eligible for PharmaCare coverage.
PharmaCare will advise the:
- Prescriber of Special Authority approval or denial.
- Pharmacy of the amount eligible for PharmaCare coverage and the specific PIN to be used when submitting the claim
SUBMITTING SAME-DAY/SAME-PHN COMPOUND PRESCRIPTIONS
If a pharmacy submits multiple claims for compounded prescriptions on the same day using the same PIN for different preparations for the same PHN, PharmaNet cannot determine that they are separate prescriptions and will reject them because it will interpret them as an error. To prevent rejection of multiple same-day/same PHN claims for compounds, submit the claim with the Intervention Code UF – Patient Gave Adequate Explanation.
Section 5.13 Tools and Resources
Section 5.14 – Insulin
General Policy Description
PharmaCare covers insulin, including biosimilars, for clients with insulin-dependent diabetes.
Policy Details
Regular insulin is reimbursed at the regular retail price with no dispensing fee.
Short-acting (rapid-acting) insulin analogues, such as insulin aspart (Trurapi®), insulin lispro (Admelog®), and insulin glulisine (Apidra®), are reimbursed at the regular retail price with no dispensing fee.
Long-acting insulin analogues such as insulin glargine (Basaglar™) and insulin detemir are reimbursed at the regular retail price, with no dispensing fee. These insulins require Special Authority approval for coverage. Refer to the Special Authority drug list.
Insulin can be dispensed to a client with or without a prescription from a prescriber.
For client safety and PharmaCare coverage, pharmacists should record insulin provided in the client's PharmaNet profile. For out-of-province/country patients, the claim does not need to be entered in PharmaNet.
If there is no prescription, the pharmacist should enter their Pharmacist ID in place of the Practitioner ID. If the pharmacist wants to enter a Prescriber ID, the pharmacist must get permission from the prescriber for the insulin to be dispensed under their Prescriber ID.
Section 5.15 – Needles and Syringes
General Policy Description
PharmaCare covers needles and syringes for patients with insulin-dependent diabetes.
PharmaCare does not cover needles and syringes for non-insulin therapy, alcohol swabs, BD glide syringes or safety needles.
Policy Details
Needles and syringes for insulin therapy
PharmaCare covers needles and syringes for insulin therapy for patients with insulin-dependent diabetes only.
Claims must be submitted using the PIN 999725 (Needles/Syringes–Insulin Use Only).
Needles and syringes for insulin therapy are reimbursed at the regular retail price with no dispensing fee.
Needles and syringes for non-insulin therapy
PharmaCare does not cover needles and syringes for non-insulin therapy (e.g., injectable heparin or dimenhydrinate).
Such claims must be entered using the PIN 66123227 (Non-Drug Medical Supplies–Non-Benefit).
Claims for needles and syringes for non-insulin use made using the benefit PIN 999725 (Needles/Syringes–Insulin Use Only) are subject to recovery by PharmaCare.
Section 5.16 – Blood Glucose Testing
General Policy Description
PharmaCare covers blood glucose test strips (BGTS), continuous glucose monitors (CGMs) and flash glucose monitors (FGMs) for eligible patients.
PharmaCare does not cover alcohol swabs, lancets, or urine test strips, except under Plan W.
In this policy, a "diabetes education centre (DEC)" is defined as a Ministry-accredited DEC operated by a health authority, and a "primary care network (PCN)" is defined as a PCN authorized by a health authority and approved by the Ministry of Health.
Blood glucose test strips (BGTS)
To be covered for BGTS, patients need to complete blood glucose monitoring training at a DEC or PCN.
People who are unable to monitor their glucose levels due to disabilities may have a caregiver complete this training on their behalf. This could be a family member, friend, or professional caregiver who monitors their levels for them. If a family member, friend, or caregiver completes the training on behalf of a patient, a Confirmation of Training in Blood Glucose Monitoring must still be faxed to HIBC.
Blood glucose monitoring training must not be connected to a community pharmacy site that submits claims to PharmaCare for BGTS or other diabetes supplies or medications. The Ministry of Health cannot accredit a DEC or approve a PCN if they oversee, share a location with, use staff that are employed by the pharmacy, or are otherwise associated with a pharmacy that make claims to PharmaCare for BGTS or other diabetes medications and supplies.
BGTS coverage is subject to annual quantity limits.
Continuous/flash glucose monitors
CGM/FGM coverage requires Special Authority (SA), and clients need to agree to diabetes education and commit to regular follow-up.
Policy Details - BGTS
Patient eligibility
PharmaCare covers BGTS for patients who meet the following conditions:
- Blood glucose testing is deemed medically necessary for the patient; and
- They have completed a blood glucose monitoring training at a DEC or PCN
- A Confirmation of Training in Blood Glucose Monitoring has been faxed to Health Insurance BC (HIBC)
Plan eligibility
- BGTS are a benefit under Fair PharmaCare, Plan C (Income Assistance), Plan F (At Home Program) and Plan W (First Nations Health Benefits).
- BGTS are not covered under Plan B since routine medical supplies are to be provided to patients at no cost by the long-term care facility. Refer to the Home and Community Care Manual, Section 6, for details.
- Using another PharmaCare plan to submit PharmaCare claims for BGTS for individuals covered under Plan B is inappropriate. Such claims are subject to recovery.
Reimbursement
- BGTS are reimbursed at their actual acquisition cost up to the PharmaCare maximum price for the product plus a dispensing fee (up to the PharmaCare maximum allowable fee).
- Consult the list of BGTS to determine the eligibility of particular strips for PharmaCare coverage and the PIN to be used to enter claims in PharmaNet.
- Pharmacists must use the specific PIN assigned to each strip when submitting a claim.
Certificates of training
DECs and PCNs submit an initial Confirmation of Training in Blood Glucose Monitoring to HIBC for their patients. Once the patient’s eligibility is entered on PharmaNet, the patient receives ongoing coverage of BGTS.
If a patient’s Confirmation of Training in Blood Glucose Monitoring has not yet been entered on PharmaNet, the patient can present a Blood Glucose Test Strip – Coverage Voucher at the pharmacy for one-time provisional coverage of BGTS (see Provisional Coverage, below).
When a patient’s eligibility for BGTS is not yet in PharmaNet and the patient presents a Coverage Voucher, the pharmacy must fax copies of both sides of the Coverage Voucher to HIBC at 250-405-3587.
Note that the First Nations Health Authority's private insurer will cover the first fill of BGTS for newly-diagnosed individuals covered under First Nations Health Benefits (Plan W), providing the BGTS is a PharmaCare benefit. For issues concerning coverage of BGTS for Plan W clients, contact the First Nations Health Benefits team at 1-855-550-5454.
Patients can contact their prescriber, DEC or PCN for information on obtaining training and certification.
>> See the procedure below for Determining if a patient has a Confirmation ("Certificate") of Training
- PharmaCare Help Desk representatives may enter provisional (1 day) coverage on PharmaNet if the pharmacy faxes copies of both sides of the Blood Glucose Test Strip – Coverage Voucher to HIBC.
- A provisional certificate is subject to a $100.00 maximum.
- Provisional coverage is provided only once for an individual.
- Provisional coverage is limited to one fill.
Quantity limits
PharmaCare applies an annual quantity limit of BGTS that will be reimbursed per patient per calendar year based on five categories of patients.
The categories are determined by the type of diabetes-related medication(s) a patient is taking, if any.
When a claim is submitted for BGTS, PharmaNet reviews all claims submitted in the previous 180 days for anti-hyperglycemic medications, whether or not the medications are covered by PharmaCare, and assigns the patient to one of five categories.
If a patient belongs to more than one category and is not using a CGM/FGM, the higher limit will apply.
Depending on a patient's medication history at the time a BGTS claim is submitted, a patient may belong to different BGTS categories within a calendar year. If a change in a patient's BGTS category occurs within the same calendar year, previous claims made during the year will be applied to their updated annual quantity limit.
All BGTS purchased, regardless of coverage, count toward a patient's annual limit.
Patients using a CGM/FGM may also need to occasionally use blood glucose test strips. For example, confirmation of blood glucose results using BGTS may be required when a patient receives an error code on their CGM/FGM or when their symptoms do not match their CGM/FGM readings.
Exceptions to the annual quantity limit for BGTS
There may be exceptional clinical circumstances in which patients need additional test strips above their annual quantity limit.
Requests for coverage of additional strips, up to the maximums indicated below, can be made through the PharmaCare Special Authority process.
Additional test strips for a pediatric patient using a CGM/FGM may be requested at the time of initial CGM/FGM request or with a CGM/FGM renewal request (using the CGM/FGM Special Authority request form).
| Patient BGTS Category | Annual Limit | Annual Exception Limit |
|---|---|---|
| Managing diabetes with insulin (no CGM/FGM) | 3,000 | No additional allowance |
| Managing diabetes with insulin and a CGM/FGM | 200 | 100 |
| Managing diabetes with anti-hyperglycemic medications with a higher risk of causing hypoglycemia† | 400 | 100 |
| Managing diabetes with anti-hyperglycemic medications with a lower risk of causing hypoglycemia‡ | 200 | 100 |
| Managing diabetes through diet/lifestyle | 200 | 100 |
†Including but not limited to insulin secretagogues (e.g., sulfonylureas, meglitinides).
‡Including but not limited to: alpha-glucosidase inhibitors (e.g., acarbose), biguanides (e.g., metformin), dipeptidyl peptidase-4 inhibitors (DPP4I), incretin mimetics/glucagon-like peptide (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors (e.g., canagliflozin), thiazolidinediones (TZDs), glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists (e.g., tirzepatide).
The Diabetes Supplies web page provides the clinical criteria for coverage of additional strips, the Special Authority request form, information on the health care practitioners who can request coverage, and instructions.
Policy Details - CGM/FGM
Patient eligibility
PharmaCare covers a continuous glucose monitor (CGM) or a flash glucose monitor (FGM) for patients who meet the following conditions:
- The patient has diabetes mellitus (DM) and meets the minimum age requirement
- For a CGM, is age 2 or older
- For a FGM, is age 4 or older
- The patient requires multiple daily injections of insulin or insulin pump therapy as part of intensive insulin therapy
- The patient, family, or caregiver agrees to comprehensive and age-appropriate diabetes education by an interdisciplinary diabetes healthcare team and commits to regular follow-up
Plan eligibility
- CGMs/FGMs are a limited coverage benefit under Fair PharmaCare, Plan C (Income Assistance), Plan F (At Home Program) and Plan W (First Nations Health Benefits)
- Starting November 7th, 2023, patients with SA approval for a CGM or FGM will receive coverage for one brand of glucose monitoring devices (e.g., Dexcom CGM system or FreeStyle Libre FGM system). Current patients will have one year to switch between devices. New patients and existing patients requesting renewals need to select one glucose monitor in their SA request
- A prescriber must submit a Special Authority request for coverage. With Special Authority in place, a CGM/FGM is fully covered
Note: Special Authority must be in place for anyone wanting PharmaCare coverage of a CGM/FGM, even if they were using a CGM/FGM before PharmaCare covered these.
Maximum days' supply per fill and dispensing interval
- CGM/FGM supplies may be dispensed in up to 90-day intervals.
- Quantity limits per 90 days
- For CGMs, patients are covered for 1 transmitter and 3 boxes of sensors (total 9 sensors); each sensor can be worn for 10 days
- For FGMs, patients are covered for 7 sensors; each sensor can be worn for 14 days
Reimbursement
- CGMs/FGMs are reimbursed at their actual acquisition cost up to the PharmaCare maximum price for the product plus a dispensing fee (up to the PharmaCare maximum allowable fee). For more information, see Section 5.7—Actual Acquisition Cost Policy.
- Consult the list of PINs to determine the eligibility of particular products for PharmaCare coverage and the PIN to be used to enter claims in PharmaNet
- Pharmacists must use the specific PIN assigned to each device component when submitting a claim
Procedures for Pharmacists - BGTS
Determining if a patient has a Confirmation ("Certificate") of Training
- Call the PharmaCare Help Desk.
- After selecting the PharmaCare Information Line option, select the Blood Glucose Certificate option.
- Enter the patient’s 10-digit PHN, and press #.
If a valid Confirmation of Training is on record, the recording will confirm this.
As an alternative to the procedure above, the pharmacist can send the transaction through on PharmaNet, and then reverse it if adjudication indicates there is no valid certificate.
SUBMITTING CLAIMS:
Submitting claims for strips within the patient’s annual limit or for which the patient or a third-party insurer will pay
Use the PIN indicated on the list as the “Regular (Within Annual Limit/Patient Pay)” PIN.
If the claim adjudication response is LO – Benefit maximum exceeded, the patient has exceeded their annual limit.
Submitting claims for strips above the patient’s annual limit
If the patient indicates they requested additional strips through their prescriber or a diabetes education centre or a primary care network, please contact the PharmaNet Help Desk to ask if Special Authority coverage is in place for additional strips.
If Special Authority is in place:
- Create a new claim with a new prescription number and use the Special Authority PIN. See Diabetes PINs.
IMPORTANT: If Special Authority is in place, but you process the claim with the regular PIN instead of the Special Authority PIN, the cost of the claim will not count toward the patient's Fair PharmaCare deductible.
If Special Authority is not in place:
- Advise the patient that they can see their prescriber or visit a diabetes education centre or a primary care network if they believe they may be eligible for approval of additional strips,
OR
- Submit the claim using the “Regular (Within Annual Limit/Patient Pay)” PIN
Submitting claims at the start of each calendar year
On January 1 of each following year, for all patients, be sure to revert to using the “regular” PINs for all patients.
Procedures for Pharmacists - CGMs/FGMs
Entering continuous/flash glucose monitors in PharmaNet
- Make sure a Special Authority is in place (1 year for initial coverage; 5 years for renewed coverage)
Submitting claims
If a Special Authority is in place:
- Create a new claim with a new prescription number
- Use the Special Authority PIN. See Diabetes PINs.
If Special Authority is not in place:
- Advise the patient to see their prescriber
Section 5.16 Tools and Resources
- Diabetes PINs web page - PINs for BGTS and CGMs/FGMs
- Special Authority criteria page for exceptions to the annual limit of BGTS
- Special Authority criteria for CGM coverage
- Special Authority criteria for FGM coverage
- Blood glucose testing web page for medical practitioners
- Diabetes supplies web page for patients
Section 5.17 – Insulin Pumps
General Policy Description
PharmaCare covers insulin pumps to ensure that cost is not a barrier to the use of an insulin pump for patients with diabetes requiring the use of regular or rapid acting insulin.
Policy Details
Patient and plan eligibility
Insulin pump coverage is available to patients who:
- Have Type 1 diabetes or another form of diabetes requiring the use of regular or rapid acting insulin, and
- Are covered under Fair PharmaCare, Plan C (Income Assistance), Plan F (Children in the At Home Program), Plan W (First Nations Health Benefits), or Plan B (Long-term Care), and
- Have been confirmed as meeting the medical criteria for coverage by their diabetes physician, and
- Have received Special Authority (SA) approval for coverage
Patients should register for Fair PharmaCare before applying for insulin pump coverage. This ensures the patient knows what their Fair PharmaCare deductible is in advance. Eligible prescription and medical device/supply costs already incurred during the year would count towards their deductible before their insulin pump purchase.
Coverage
PharmaCare coverage is limited to one insulin pump every five years (or after four years if the current pump was previously covered by an organization other than PharmaCare, such as a private insurer).
Only the makes and models of insulin pumps approved by PharmaCare are eligible for PharmaCare coverage, and only when the insulin pump is purchased from an approved vendor, as identified for the patient and their endocrinologist in their SA approval confirmation letter.
Coverage will not exceed the PharmaCare maximum price for a particular insulin pump make and model, with no dispensing fee.
The Omnipod Insulin Management System personal diabetes manager is available at no cost regardless of the patient’s PharmaCare plan. The YpsoPump is available at no cost regardless of PharmaCare plan with the purchase of a Ypsomed starter kit, which is supplied at $800. The MiniMed Insulin Pump System is supplied at $6,600.
Coverage of the Ypsomed starter kit and the MiniMed Insulin Pump system is subject to the rules of the patient’s PharmaCare plan. Coverage is as follows:
- If the patient is covered under Plan B (Long-term Care), Plan C (Income Assistance), Plan F (Children in the At Home Program), or Plan W (First Nations Health Benefits), PharmaCare covers 100% of the costs
- If the patient is covered under Fair PharmaCare, the cost is subject to the patient’s deductible and family maximum. PharmaCare covers 70% of the costs once the patient has met their deductible. PharmaCare covers 100% of the costs once the patient has met their family maximum
Important: Patients who are not covered by a PharmaCare plan that pays 100% should register for Fair PharmaCare before purchasing an insulin pump to ensure they receive maximum coverage.
Coverage requirements
PharmaCare coverage of insulin pumps requires prior Special Authority approval by PharmaCare. Approval is provided on a case-by-case basis.
Special Authority approval for insulin pump coverage cannot be provided retroactively.
Special Authority approval for the purchase of an insulin pump may be requested once every five years on behalf of an eligible patient. Special Authority requests must be submitted by the referring specialist physician or endocrinologist.
The Insulin Pump Special Authority web page provides the medical criteria for coverage, the Special Authority request form, and instructions about how to secure coverage.
PharmaCare sends a letter confirming or denying coverage to the referring specialist physician or endocrinologist. The physician must provide a copy of the approval letter to the patient for use in purchasing the approved insulin pump.
The patient must provide a copy of the Special Authority approval letter to the insulin pump vendor prior to or at the time of purchase.
Insulin pump claims for patients who do not have PharmaCare Special Authority approval will not be paid by PharmaCare.
Patients with existing insulin pumps not covered by PharmaCare
Patients with an existing insulin pump that was not covered by PharmaCare may be eligible for PharmaCare coverage if:
- They meet the patient and plan eligibility criteria,
- They meet the medical criteria for a subsequent insulin pump, and
- Their current pump is four or more years old, and
- The manufacturer's warranty for their current pump has expired
The patient’s specialist physician or endocrinologist must submit a Special Authority request to PharmaCare requesting coverage.
The patient must contact their insulin pump manufacturer for a letter confirming the warranty expiry date. The physician must include this proof of warranty expiry with the Special Authority request.
Reimbursement
Insulin pumps are reimbursed at the retail price up to the PharmaCare maximum allowable cost for the pump, with no dispensing fee.
Insulin pump repairs and replacement
PharmaCare does not cover insulin pump repairs.
PharmaCare does not cover insulin pump replacement prior to the end of the five-year period since coverage for a patient’s last pump was issued.
Insulin pump repairs and/or replacement of broken pumps are subject to the terms of the manufacturer’s warranty during the warranty period. The patient should refer all enquiries about pump repair and replacement to the vendor from which the pump was purchased.
Lost or stolen insulin pumps
Replacement costs for stolen or lost insulin pumps are not covered by PharmaCare.
Information for insulin pump vendors
A copy of the PharmaCare letter confirming Special Authority approval for insulin pump coverage must be obtained from the purchaser and maintained on file. This letter will identify the model of pump that the patient has coverage for.
Insulin pump claims for patients who do not have PharmaCare Special Authority approval for insulin pump coverage will not be paid by PharmaCare.
Insulin pump vendors must use the correct Product Identification Number (PIN) for the insulin pump that the patient has been approved for, as identified in the Special Authority confirmation letter.
Information on connection to PharmaNet and online claims payment processes, as well as the processing of manual, paper-based PharmaCare claims, is contained in the PharmaCare Claims for Insulin Pump Vendors Quick Guide (PDF, 1.2 MB).
Questions and Answers
What if I encounter problems using a pump instead of regular injections?
Speak to your endocrinologist or diabetes specialist. If, in consultation with your specialist, it is determined that you cannot continue using a pump, you may be able to return it. Vendors may allow you to return the pump for a refund within 90 days of purchase. In this case, the vendor will reverse the PharmaCare claim and refund any portion of the cost you paid.
What if my pump stops working after the five-year period is up?
If your pump is beyond economical repair, contact the vendor of your pump and ask for a letter confirming your warranty expiry date. Take the letter to your endocrinologist or specialist physician, who will include the letter with a new Special Authority request to PharmaCare.
Section 5.17 Tools and Resources
Section 5.18 – Insulin Pump Supplies
General Policy Description
PharmaCare covers insulin pump infusion sets/kits and reservoirs/cartridges. PharmaCare does not cover other insulin pump supplies such as batteries, battery caps, adhesive pads, and pump covers.
Policy Details
Patient and plan eligibility
PharmaCare covers insulin pump infusion sets/kits and reservoirs/cartridges for patients if they are covered under:
This coverage is available whether or not the cost of the insulin pump was covered by PharmaCare.
PharmaCare Special Authority pre-approval is not required for insulin pump supplies.
Supplies covered by PharmaCare
PharmaCare covers only the pods, infusion sets/kits and insulin pump reservoirs/cartridges listed on the Insulin Pump Supplies page.
PharmaCare does not cover other insulin pump supplies, such as batteries, battery caps, adhesive pads, pump covers, etc.
Purchasing and reimbursement
PharmaCare reimburses claims for eligible insulin pump supplies purchased from pharmacies and approved insulin pump vendors who submit medical supply claims on PharmaNet. PharmaCare does not accept paper/manual claims for insulin pump supplies.
- Exceptions may apply for patients living in border communities who are served by an out-of-province pharmacy that participates in PharmaCare
Insulin pump supplies are reimbursed at the retail price up to the PharmaCare maximum allowable cost for the product with no dispensing fee.
Pharmacies and insulin pump supply vendors must use the correct Product Identification Number (PIN) and unit of measure for the dispensed quantity when entering claims in PharmaNet.
>> For correct quantity information and insulin pump supply PINs, see Insulin Pump Supplies.
Section 5.18 Tools and Resources
Section 5.19 – Reimbursement for Non-Returnable High-Cost Injectable Drugs
General Policy Description
PharmaCare allocates an annual pool of funds to provide reimbursement for the adjudicated PharmaCare-paid ingredient cost for an eligible high-cost injectable drug that was ordered for a specific patient but, due to circumstances outside the control of the pharmacy, was not received by the patient.
Reimbursement is subject to the eligibility of a drug as determined by PharmaCare, the availability of funds, and the specific conditions defined below under Reimbursement Policy and Conditions.
Policy Details
Funding and payments
Funds allocated for reimbursement each fiscal year will equal 0.25% of total PharmaCare expenditures in the preceding fiscal year for the list of drugs to which this program applies.
If the total cost of reimbursements under this program exceeds the funds available for a particular quarter, reimbursements will be pro-rated based on the proportion of total claimed reimbursements that an individual claim represents.
Funds are allocated evenly across quarters (that is, 25% of the total funds per quarter). Any available funds that are not distributed in a given quarter are carried forward to the following quarter. Any funds not distributed at the end of the fiscal year will be retained by the Province.
Drugs eligible for reimbursement
The drugs eligible for reimbursement under this policy are listed on the Reimbursement for Non-returnable High-cost Injectable Drugs–Eligible Products List (PDF, 360 KB).
Reimbursement policy and conditions
A pharmacy may submit a claim for compensation for a reversed claim for an eligible high-cost injectable drug if all the following conditions are met:
- The original claim was submitted to PharmaCare and the PharmaCare-paid ingredient cost amount was greater than $0.00.
- The drug was ordered to fill a prescription for a specific patient. After the pharmacy ordered the drug, the patient’s treatment was terminated or suspended or the patient was otherwise unable to take delivery of the drug.
- The pharmacy had no opportunity to dispense the drug to another patient or return the drug for refund.
- The pharmacy has not submitted a previous claim for compensation of a reversed eligible high-cost injectable drug claim for the same patient within the same fiscal year (April 1 – March 31).
- Product loss due to theft, fraud, handling error, equipment or power failure, Act of God or other cause not specifically mentioned above is not eligible for reimbursement under this policy.
- The pharmacy must submit a claim for reimbursement by reversing the claim on PharmaNet within 30 days of the patient’s service date for the original claim and use the intervention code specifically assigned for this program.
No retroactive payments can be made for omission of the intervention code or for reversals submitted more than 30 days after the date of the original claim.
Reimbursements will be paid by a financial adjustment to the pharmacy’s payment following the end of the quarter in which the claim is reversed.
Procedures
PROCEDURES FOR PHARMACISTS:
Claiming reimbursement for an eligible high-cost injectable drug
Reverse the original claim using the intervention code NR – Non-returnable Drug Reimbursement.
Note that when you submit a reversal with the NR code, PharmaNet appears to process the reversal as a regular reversal—there will be no indication of compensation. This is because NR–Non-returnable Drug Reimbursement reversals are held and processed in a batch once every quarter. Since no financial transaction takes place at the time of submission, no payment message is returned.
Section 5.19 Tools and Resources
Section 5.20 – Smoking Cessation Program Policy
General Policy Description
The Smoking Cessation Program covers smoking cessation products for eligible B.C. residents of any age who wish to stop smoking or using other tobacco products.
Individuals are covered for eligible prescription smoking cessation drugs under the rules of their primary PharmaCare plan (including any deductible or family maximum).
Eligible nicotine replacement therapy products are provided at no cost to all eligible individuals regardless of the rules of their primary PharmaCare plan.
Policy Details
GENERAL POLICIES:
Smoking cessation products covered
The Smoking Cessation Program covers two types of smoking cessation products:
- Prescription smoking cessation drugs—bupropion (brand name Zyban®) and varenicline (generic brands)
- Specific non-prescription nicotine replacement therapy (NRT) products
Please see the specific NRTs covered in Products Covered.
Duration and frequency of coverage
Coverage is limited to a single continuous course of treatment lasting up to 12 weeks (84 days) of one eligible smoking cessation product (i.e., one course of a nicotine replacement therapy, or bupropion, or varenicline) each calendar year.
The 12 weeks (84 days) of coverage begins on the day of the first fill of the smoking cessation product.
All eligible fills of the product must be dispensed within 84 days of the first fill.
The Smoking Cessation Program coverage year runs from January 1 through December 31. Unused coverage from one calendar year cannot be carried over into the next calendar year.
Starting January 1 of every year,
- A new coverage year begins and each patient’s existing previous coverage is cancelled, even if the patient is only part way through the 12-week course of treatment; and
- Coverage is reset to 84 consecutive days (12 weeks) for the new year.
Patients who have not received all their eligible product fills for a course of treatment by December 31 may continue their treatment into the next year by accessing their new 84 days of coverage for the next year.
PharmaCare recognizes that the pack size of a particular product may result in minor overruns in treatment duration (e.g., six days over the course of treatment for blister-packed products in a 30-day supply pack size), and these small overruns are deemed acceptable.
Supplemental coverage or changes in coverage
Individuals who want to supplement the coverage available through the Smoking Cessation Program are expected to cover the costs themselves or through their extended health plans.
Under exceptional and compelling circumstances, PharmaCare may permit a change in the currently covered course of treatment (e.g., a change from a nicotine replacement therapy to one of the prescription smoking cessation drugs or vice versa).
To request a change in the currently covered course of treatment, the prescribing physician must submit a General Special Authority Request form (PDF, 656KB) requesting exceptional case-by-case consideration.
The request must include:
- The patient’s diagnosis
- The current smoking cessation therapy
- Benefits that would accrue from changing the current course of treatment
- Reasons for the change
- Length of treatment needed
- Name and dosage of the alternate product, if applicable
Special Authority approval is not provided retroactively.
Claims for Plan B patients
Plan B patients are eligible for a prescription smoking cessation drug or nicotine replacement therapy (NRT).
Since PharmaCare pays a monthly capitation fee to contracted pharmacies in addition to eligible drug costs for Plan B patients, no dispensing fees can be claimed for prescription smoking cessation drugs or non-prescription NRTs dispensed to Plan B patients.
Claims for bupropion (Zyban®) and varenicline generics should be processed in the usual fashion for Plan B patients.
To ensure NRT claims for Plan B patients adjudicate to Plan S (under which they are a benefit) and not Plan B (under which they are not a benefit), the pharmacy must ensure that the long-term care facility code is not entered in NRT claims submitted on PharmaNet.
B.C. residents covered by federal drug plans
As with other prescription medications, PharmaCare is not the first payer for prescription smoking cessation drugs. However, federally insured patients with active Medical Services Plan coverage can choose PharmaCare coverage for NRTs, even if their federal insurer provides coverage. This includes individuals insured under
- Veterans Affairs Canada
- Non-Insured Health Benefits Program
- RCMP (non-retired members)
- Interim Federal Health Program (delivered through Immigration, Refugees and Citizenship Canada)
To process a Smoking Cessation Program claim for an individual covered under a federal drug plan, please see Procedures for Pharmacists.
Medication review eligibility
Smoking cessation prescription drugs dispensed under the Smoking Cessation Program count as qualifying medications for purposes of determining a patient’s eligibility for medication review services.
>> See the Section 8.9—Medication Review Services for further information
Partial fills due to product shortages
If a pharmacy cannot provide a patient with the full amount of their prescription smoking cessation drug or nicotine replacement therapy, pharmacists should enter only one claim for the full amount, and ask the patient to return for the balance.
If a patient does not return to pick up the balance, pharmacists must adjust their claim to reflect the amount actually dispensed and picked up.
Pharmacies cannot substitute a non-benefit product for those listed as benefits under the Smoking Cessation Program.
NICOTINE REPLACEMENT THERAPIES:
Eligibility
Coverage is available to all smokers (and users of other tobacco products) of any age who are B.C. residents with active Medical Services Plan (MSP) coverage.
To enroll, both the individual and pharmacist must sign a BC Smoking Cessation Program Declaration and Notification form (HLTH 5464) (PDF, 560KB).
PharmaCare coverage of nicotine replacement therapies is limited to the products in the list of Eligible Smoking Cessation Products.
Claims for nicotine replacement therapies must be submitted with the applicable NPN.
Important: Please note that only the package sizes specified are eligible for coverage under the program. Claims for package sizes not specified in the list of Eligible Smoking Cessation Products above are subject to recovery upon audit.
Access to nicotine replacement therapies
Eligible individuals receive no-cost (100%) coverage of the designated nicotine replacement therapies (NRTs) purchased at a pharmacy in the same manner as prescription drugs.
Patients do not need a prescription for NRT coverage.
Both the patient and the pharmacist must sign a BC Smoking Cessation Program Declaration and Notification form (HLTH 5464) (PDF, 560KB).
Claims without a corresponding signed declaration are subject to recovery upon audit.
Pharmacies must submit a claim on PharmaNet at the time of purchase to access PharmaCare coverage for NRTs for their patients.
A new claim must be entered in PharmaNet for each NRT fill.
NRT coverage is not subject to, and does not contribute to, the Fair PharmaCare annual deductible or family maximum.
Maximum days' supply per fill and dispensing interval
Nicotine patches:
Eligible nicotine patches are to be dispensed in four-week (28-day) intervals.
PharmaCare limits coverage of eligible nicotine patches to a maximum 28-day supply.
Patients are covered for four boxes of patches (total 28 patches) every 28 days.
Over the total 12-week (84-day) course of treatment, patients are eligible for coverage of up to 84 NRT patches (supplied as 12 boxes with seven patches in each box). This quantity is based on the maximum dosing specified in the product monograph.
Nicotine gum:
Over the total 12-week (84-day) course of treatment, patients are eligible for up to 945 pieces of nicotine gum (supplied as nine boxes with 105 pieces in each box). On average, most patients need three boxes of nicotine gum (total 315 pieces) every 28 days. The maximum per fill is 3 boxes.
Nicotine lozenges:
Over the total 12-week (84-day) course of treatment, patients are eligible for up to 800 pieces of nicotine lozenge (supplied as 10 bottles with 80 lozenges in each bottle). One fill can be for 4 boxes, but the other 2 fills cannot exceed 3 boxes.
Changing nicotine replacement therapy products during a course of treatment
During a course of treatment, patients may switch between different types of nicotine replacement therapy—or from one dosage strength to another—only when picking up one of the three fills covered for the course of treatment. Such changes do not require Special Authority approval.
All Smoking Cessation Program policy limitations with respect to maximum days’ supply, dispensing intervals and dispensing fees continue to apply regardless of changes in product or strength.
Any changes in product or strength that may create a dispensing that is not in compliance with the Smoking Cessation Program policy limitations require prior Special Authority approval.
Dispensing fees
Community pharmacies are reimbursed for a dispensing fee up to the PharmaCare maximum for the dispensing of eligible nicotine replacement therapies (NRTs).
PharmaCare covers the dispensing fee for up to three dispenses per patient per course of treatment with one of the designated NRTs.
The Frequency of Dispensing Policy applies to NRTs.
PharmaNet cannot automatically reject NRT-related dispensing fees above the maximum allowable three dispensing fees. PharmaCare requires pharmacies to check a patient’s PharmaNet record to ascertain the number of previous NRT fills a patient has had and to ensure no more than three dispensing fees are claimed per patient per course of treatment.
Clinical services fees
Nicotine replacement therapies are not eligible for clinical services fees.
Special services fees
Nicotine replacement therapies are not eligible for special services fees.
Application of the Full Payment Policy
The Full Payment Policy applies to all PharmaCare paid nicotine replacement therapy claims since they are covered 100% in all cases. Therefore, pharmacies may not charge individuals directly for any amount in excess of the PharmaCare paid amount for nicotine replacement therapy claims covered under the Smoking Cessation Program.
GST reimbursement for nicotine replacement therapies
Nicotine replacement therapies (NRTs) and fees to dispense them are subject to 5% GST.
The GST must not be included as part of the claim for either the NRT product or the dispensing fee.
The GST must not be collected directly from either the patient or a third-party insurer.
The provincial government reimburses pharmacies for the GST associated with NRT claims paid under the Smoking Cessation Program. Pharmacies are reimbursed on a quarterly basis.
Each pharmacy’s NRT claims are reviewed and pharmacy payments are adjusted to reimburse 5% for the GST.
Returns or exchanges of nicotine replacement therapies
Patients cannot return any unused products for exchange, reimbursement or credit.
SMOKING CESSATION DRUGS:
Eligibility
Coverage is available to all smokers (and users of other tobacco products) of any age who are B.C. residents and are registered for Fair PharmaCare or Plan B (Long-term Care), Plan C (Recipients of Income Assistance), Plan G (Psychiatric Medications) or Plan W (First Nations Health Benefits).
Products covered
PharmaCare coverage of prescription smoking cessation drugs is limited to certain eligible products. (See list of Eligible Smoking Cessation Products).
Of the different versions of bupropion, PharmaCare covers only Zyban® for smoking cessation. Wellbutrin®, Wellbutrin XL® and generic bupropion are not covered as smoking cessation drugs.
To ensure the prescription is for the version of bupropion eligible for Smoking Cessation Program coverage, prescribers are to indicate on the prescription
- The brand name Zyban®
- The prescription is for “smoking cessation”
- “No substitutions”
PharmaCare only fully covers generic versions of varenicline. Brand name varenicline (Champix®) is a partial benefit.
Coverage
Coverage is subject to the applicable PharmaCare plan rules. Plans B, C, G and W provide 100% coverage. Fair PharmaCare provides partial, full or no coverage depending on whether an individual has met their annual deductible and family maximum.
Coverage does not require a request for Special Authority; however, Limited Coverage criteria should be used to assess patient eligibility for coverage.
>> See the Limited Coverage Drug Program Criteria Information for details.
Patients require a prescription to be eligible for coverage.
Individuals covered by Fair PharmaCare who receive eligible prescription smoking cessation drugs but who do not receive PharmaCare coverage in whole or part because they have not met their Fair PharmaCare deductible, are eligible for certain free eligible nicotine replacement therapies for that same calendar year.
Individuals covered by Fair PharmaCare who receive PharmaCare coverage in whole or part for an eligible prescription smoking cessation drug are not eligible for coverage of nicotine replacement therapies in that same calendar year.
Maximum days' supply per fill and dispensing interval
Eligible prescription smoking cessation drugs are to be dispensed in four-week (28-day) intervals.
PharmaCare covers a maximum 28-day supply of eligible prescription smoking cessation drugs.
Dispensing fees
Except as noted below, PharmaCare covers the dispensing fee for up to three dispenses per patient (not per pharmacy) per course of treatment with an eligible prescription smoking cessation drug.
When a 14-day starter pack of varenicline (generics) is dispensed patients may receive four fills of medication (two in the first 28 days, followed by two additional fills of 28 days each) and pharmacies may claim up to a maximum of four dispensing fees per patient per course of treatment.
If specified on the prescription, PharmaCare may cover more frequent dispenses over the course of treatment up to a maximum specified in Section 8.3—Frequency of Dispensing Policy.
Clinical services fees
Prescription smoking cessation drugs are eligible for clinical services fees for dose and regimen changes but not for prescription renewals or therapeutic substitutions.
Special Services Fees
Prescription smoking cessation drugs are eligible for Special Services Fees.
Application of the Full Payment Policy
The Full Payment Policy applies to prescription smoking cessation drug claims in the same manner as any other prescription drug claim.
Submitting claims for nicotine replacement therapies
- Confirm in PharmaNet that the patient has received PharmaCare coverage for no more than two previous nicotine replacement therapy (NRT) fills in the current calendar year, with respect to the 84-day course of treatment. If the patient has had three fills in the current calendar year, explain to them that they have used up their coverage for the current year.
Check PharmaNet not your local pharmacy system since a patient may have had NRTs dispensed through a different pharmacy.
- Ensure that the Declaration and Notification form (HLTH 5464) (PDF, 560KB) is fully completed; that is, ensure all required fields are completed and that both you and the patient have signed the form.
A declaration form must be signed for each NRT fill or refill. - In separate yearly files, file the declaration form by patient name, then chronologically. Claims without a corresponding signed declaration form or with only one signature are subject to recovery upon audit.
- Enter the fill as a new prescription (not a refill).
- Enter the appropriate product NPN.
- Enter your pharmacist College ID in the Practitioner ID field.
- Enter the number of units dispensed in the Dispensed Quantity field (i.e., the number of pieces of gum, lozenges or patches).
- Enter the drug cost and dispensing fee. Do not add GST to either the drug cost or the dispensing fee.
Advising the patient about subsequent fills
If a patient has refills remaining, remind them to wait at least two weeks before refilling. This ensures their refill is within the limits of the Refilling Prescriptions Too Soon policy.
Submitting claims for prescription smoking cessation drugs
- Confirm that the prescribed drug is eligible for Smoking Cessation Program coverage.
Prescriptions for varenicline (Champix® and generics) can be written in the usual fashion.
Prescriptions for bupropion must specify the Zyban® brand of bupropion and indicate the prescription is for smoking cessation.
- Review the patient’s medication history in PharmaNet to determine if the patient has already received prior PharmaCare coverage for a course of treatment with a smoking cessation product (nicotine replacement therapy, bupropion or varenicline) in the current calendar year or has used up their coverage for a current course of treatment.
Note that you must use PharmaNet rather than your local system as a patient may have had smoking cessation products dispensed by another pharmacy. These fills count towards the total allowable for the patient.
- Submit the claim.
If a patient has refills remaining, remind them that, to receive PharmaCare coverage, they cannot refill their prescription until they have a remaining supply of 14 days or less.
Submitting claims for patients with federal drug plan coverage
Patients with federal drug coverage may request coverage under the Smoking Cessation Program even if they have existing smoking cessation aid coverage under their federal plan.
Individuals choosing coverage under the Smoking Cessation Program are subject to all policies and procedures of the program.
Submit the claim as described in the Claims for Prescription Smoking Cessation Drugs and Claims for Nicotine Replacement Therapies sections above.
When a patient covered by a federal drug plan requests NRTs or presents a prescription for one of the smoking cessation drugs covered by the program:
- Submit the claim to the patient's federal plan first.
- If the federal plan indicates it will not cover the product for the patient, then
- Remove the federal coverage in your local system.
- Call the PharmaCare Help Desk to have the federal coverage flag removed, so that
- the NRT claim can adjudicate as PharmaCare pays 100%, or
- the prescription smoking cessation drug claim can adjudicate according to the rules of the patient's PharmaCare plan.
3. If the federal plan claim indicates it will cover the product, the patient can choose PharmaCare coverage instead.
- Ensure the patient understands that, while NRTs will be free of charge, the prescription drugs may not (depending on the rules of the patient's primary PharmaCare plan, including any deductible requirements).
- For example, patients covered by Fair PharmaCare, due to annual deductibles, receive more coverage under their federal plan for a prescription smoking cessation drug.
4. If the patient chooses PharmaCare coverage, remove federal coverage as established in Step 2 above.
Section 5.20 Tools and Resources
Visit the appropriate Smoking Cessation Program website for more information:
Section 5.21 – Ostomy Supplies
General Policy Description
PharmaCare covers certain ostomy supplies for patients who have undergone surgery on the bowel and/or bladder that results in a colostomy, ileostomy or urostomy, requiring the application of an external pouch.
Policy Details
PharmaCare covers eligible ostomy supplies for patients who have undergone surgery on the bowel and/or bladder that results in a colostomy, ileostomy or urostomy, requiring the application of an external pouch.
Coverage is subject to the rules of the patient's primary PharmaCare plan, including any annual deductible requirements.
PharmaCare covers eligible ostomy supplies up to the regular retail price with no dispensing fee.
Claims must be entered into PharmaNet using the Product Identification Number (PIN) indicated in the list of eligible ostomy supplies.
Other ostomy supplies, including but not limited to the following, are not PharmaCare benefits:
- Catheters—for any use
- Ostomy support belts
- Pouch covers
- Night drainage bottle covers
- Stoma hole cutters
- In-pouch deodorants—such as Banish, M9, Uri-Kleen
- Cleansers—such as Hollister Restore Wound Cleanser, Uni-Wash, ConvaTec AloeVesta products
- Room deodorants—such as M9
- Tapes (other than paper-type)—such as Elastoplast, Dermicel, Waterproof, 3M Blenderm
- Creams—such as Sween Cream, Chiron Cream, BAZA
- Lubricants—such as KY Jelly, Hollister Stoma Lubricant
- Products for management of incontinence—such as catheters, condoms, Attends, drainage containment equipment
- Hydrocolloid dressings—such as DuoDerm, Restore, Tegasorb
- Transparent dressings—such as Opsite, Tegaderm
- Sterile/unsterile gauze
- Alcohol swabs
- Products for the management of feeding tubes and draining wounds—such as Hollister Drain Tube Attachment Device, Hollister Drainage Collectors
- Instruments—such as scissors, dressings sets
Section 5.22 – Prosthetics and Orthotics
General Policy Description
The Prosthetic and Orthotic Program helps patients to achieve or maintain basic functionality.
PharmaCare helps eligible patients pay for the costs of eligible prostheses and orthoses, subject to the rules of their PharmaCare plan, including any annual deductible requirement.
Policy Details
Full details of the Prosthetic and Orthotic Program and associated policies are provided in the Prosthetic and Orthotic Policy Manual.
Section 5.22 Tools and Resources
Section 5.23 – Pricing Exceptions Where Multiple Dosage Forms or Strengths are Available
General Policy Description
To ensure the best value is obtained for expenditures on drugs available in multiple dosage forms and/or strengths, PharmaCare may limit reimbursement of a particular dosage form or strength to the cost of a lower cost dosage form and/or strength.
Policy Details
PharmaCare may limit reimbursement of a particular dosage form or strength to the cost of a lower, or the lowest, priced dosage form and/or strength of a drug.
For example,
- If the price of the capsule, sustained release or enteric-coated tablet is significantly higher than the price of the tablet, prices may be based on that of the tablet.
- If a product strength is priced significantly higher (proportionally) than the price of other strengths, prices may be pro-rated based on that of the lowest cost strength.
- If the price of the suspension is significantly higher than the price of the solution, prices may be based on that of the solution.
- For creams and ointments, if the per-unit price of the tube is significantly higher than the per-unit price of the jar, prices may be based on that of the jar.
To determine the specific pricing exceptions in force under this policy, refer to Low Cost Alternative (LCA) Program.
Section 5.24 – Drug Shortages
General Policy Description
PharmaCare may decide to cover an alternative drug when a benefit drug is in short supply. When a shortage ends, the alternative product returns to its former benefit status.
Policy Details
Drug shortages occur when a manufacturer or supplier cannot provide enough of a drug to fulfill demand. This could be due to issues such as manufacturing disruptions, shipping delays, shortages of the active or non-active pharmaceutical ingredients, or sudden increases in demand.
PharmaCare may cover an alternative drug when a benefit is in short supply. Coverage for the alternative drug is often the same as for the drug in short supply – i.e., covered under the same plan(s), with the same Special Authority requirements (if any).
PharmaCare may, on a last-resort basis, cover a compounded drug as the alternative drug.
If PharmaCare is not covering an alternative drug during a shortage
PharmaCare often covers several versions of a drug. If one manufacturer’s version runs short, prescribers and pharmacists may be able to substitute another, comparable product from a different manufacturer. The Low Cost Alternative program provides information about drugs that are interchangeable within the same category.
If PharmaCare decides to not substitute the drug in short supply with another covered product, patients may need to discuss treatment options with a health care provider.
Where to find drug shortages information and covered alternatives
PharmaCare’s Drug shortages web page is continually updated with essential information about drug shortages, including covered alternatives. The page links to:
- Current Drug Shortages List (xls), which provides details about PharmaCare benefits that are in short supply and alternatives that PharmaCare is covering. The list does not include drugs used in hospitals
- Resolved Shortages List (xls), which provides details about drug shortages that have ended
- Drug Shortages Canada, a web page that includes a complete list of all reported shortages and discontinuations
Resources
- Drug shortages web page – includes the Current and Resolved drug shortages lists
- PharmaCare Policy Manual Section 5.13—Compounded Prescriptions
- Pharmacists or prescribers may contact the HIBC Help Desk with questions about coverage for alternative drugs

6 – Understanding PharmaCare Benefit Status
Section 6.1 – Benefit Status Types
General Policy Description
PharmaCare uses an evidence-informed approach to drug policy development. By confirming that clinical evidence supports a drug or medical supply or device’s effectiveness before considering it for coverage, PharmaCare ensures the wise use of program resources.
Policy Details
PharmaCare considers a drug or medical device or supply for coverage upon receiving a request by the drug's manufacturer. After completing its review, PharmaCare decides whether a drug will be a full benefit, a partial benefit, a limited coverage benefit (covered only if a patient meets specific criteria) or a non-benefit (not covered).
Benefit status definitions
Each prescription drug or eligible medical supply or device is assigned one of four PharmaCare benefit status types:
|
Benefit Status |
Coverage Details |
Notes |
|---|---|---|
|
Regular benefit |
Eligible for full reimbursement subject to PharmaCare price limits and subject to the rules of a patient’s PharmaCare plan. |
|
|
Partial coverage |
Eligible for limited reimbursement under the Low Cost Alternative Program or the Reference Drug Program or other maximum, and subject to the rules of a patient’s PharmaCare plan. |
In some situations, PharmaCare may grant full coverage through Special Authority to a drug that would otherwise be only a partial benefit. |
|
Limited Coverage drug |
Eligible for reimbursement only in certain medical circumstances and subject to the rules of a patient’s PharmaCare plan. Before a patient can get PharmaCare coverage, their prescriber must submit a Special Authority request to PharmaCare. |
The drug is usually a second-, third- or fourth-line treatment. Drug is subject to LCA program rules if a low-cost alternative exists. |
|
Non-benefit |
Not eligible for PharmaCare coverage. Special Authority is available only on an exceptional, last-resort basis, generally when all available PharmaCare benefit options have been tried without success or are unsuitable for the patient and no other coverage options are available (e.g., third-party insurance). If the drug subsequently becomes a benefit, PharmaCare cannot provide retroactive coverage. |
For a list of non-benefits, see What BC PharmaCare does not cover. |
- A prescription medication or medical supply may have a different benefit status under different PharmaCare plans.
- Patients wishing to take a partial benefit will be required to pay the difference between the Low Cost Alternative (LCA) or Reference Drug Program (RDP) price and the full cost of the prescription. For Fair PharmaCare patients, only the lower amount counts toward the annual deductible and family maximum, unless Special Authority has been provided.
Procedures for Pharmacists
Determining benefit status
There are several ways to learn the benefit status of a drug or supply:
- The online BC PharmaCare Formulary Search.
- The PharmaCare Newsletter routinely provides information on the completion of reviews, new benefits, and changes in benefit status.
- The Drug Review Results web page.
- Pharmacists may contact the PharmaCare Help Desk and choose either the interactive voice-response line or hold to speak to a representative.
- Some in-pharmacy software stores benefit/non-benefit information.
Section 6.1 Tools and Resources
Section 6.2 – Health Canada's Special Access Program Drugs
General Policy Description
Physicians occasionally treat patients with medications not approved for sale in Canada in cases of serious or life-threatening illness when conventional therapies have failed, are unsuitable, are unavailable, or offer limited options.
The Therapeutic Products Program (TPP) of Health Canada is mandated to authorize the sale of these medications to physicians. The Special Access Program (SAP) of the TPP administers this mandate. SAP is responsible for authorizing the sale of pharmaceutical, biologic and radio-pharmaceutical products that are not approved for sale in Canada.
The prescribing physician is required to submit an application to the SAP for approval to use an SAP drug for a patient. Once approved, the SAP authorizes release of the drug to the physician.
Policy Details
PharmaCare coverage of SAP drugs
PharmaCare coverage for SAP drugs is available only under exceptional circumstances through the Special Authority process.
PharmaCare otherwise does not cover non-approved indications or drugs that have not been approved for sale in Canada.
Special Authority approval must be in place before the drug is dispensed to the patient. Retroactive coverage cannot be provided.
Procedures for Physicians and Pharmacists
Applying for coverage of an SAP drug
A prescribing physician must submit an application to the SAP for approval to use an SAP drug for a patient. Once approved, the SAP sends a notification to the manufacturer and a copy to the physician, authorizing the release of the drug to the physician. The manufacturer then supplies the drug directly to the physician or to a hospital pharmacy. (Manufacturers cannot release SAP drugs directly to community pharmacies.)
Because SAP medications are not approved for sale in Canada, they do not have drug identification numbers (DINs). In order for a pharmacy to dispense an SAP drug, PharmaCare will assign a Product Identification Number (PIN).
The pharmacist can request a PIN by providing the drug information (including manufacturer, generic name, brand name and dosage form) to PharmaCare.
If PharmaCare grants an individual patient Special Authority coverage for an SAP drug, a confirmation letter is sent to the patient's physician indicating the PIN for that drug. SAP approval does not guarantee PharmaCare will cover the SAP drug.
If the physician does not advise the pharmacist of the PIN to be used, the pharmacist can call the PharmaCare Help Desk to obtain it.
If PharmaCare has granted Special Authority coverage for an SAP drug, a claim must be entered on PharmaNet by a community or hospital pharmacy.
Physicians are advised to make arrangements with the hospital pharmacy or local community pharmacy before the SAP drug is received.
For instance, the hospital or community pharmacy may agree to purchase the drug from the physician. The pharmacy can then dispense the drug and enter the prescription details on PharmaNet. In addition to ensuring appropriate coverage by PharmaCare, this also ensures that the prescription information is included on the patient's medication profile. Please note that the physician would remain responsible for the manufacturer's invoice and should remit payment directly to the manufacturer. PharmaCare cannot reimburse physicians or patients directly.
Section 6.2 Tools and Resources
SAP contact information
Telephone: 613-941-2108 (8:30 am-4:30 pm Eastern Time)
Fax: 613-941-3194
E-mail: sapd-pasm.sc@canada.ca
Section 6.3 – Special Authority Coverage
General Policy Description
For some drugs, PharmaCare requires the patient’s health care provider to submit a request for Special Authority (SA) approval in order to be eligible for coverage.
In these cases, PharmaCare coverage is applicable only to prescriptions purchased after Special Authority has been granted and entered in PharmaNet. No retroactive coverage is available.
Policy Details
Patient Special Authorities
Special Authority approval grants full or partial PharmaCare coverage for a drug that might otherwise not be covered or be covered only partially.
The rules of a patient's plan, including any deductible and co-payment requirement, apply even if the patient is granted Special Authority coverage.
Special Authority approval does not exempt the drug from PharmaCare pricing policies such as the PharmaCare Maximum Pricing Policy, the Low Cost Alternative Program or the Reference Drug Program, unless the Special Authority has been specifically granted for that purpose.
Special Authority coverage cannot be provided retroactively. Special Authority approval must be in effect on PharmaNet when the patient purchases the prescription.
Special Authority may be granted for
- A Limited Coverage medication
- An alternate product for patients unable to use the low-cost alternative (due to allergy) or reference drug product (due to adverse reaction or treatment failure)
- Drugs that are not marketed in Canada (e.g., Health Canada Special Access Program Drugs), in exceptional circumstances only
Although PharmaCare Special Authority Requests are normally approved only for patients who meet established criteria, under exceptional circumstances, PharmaCare may cover patients who do not meet the pre-defined criteria when a request is made by an appropriate health care practitioner.
Special Authority approval for groups of similar medications
PharmaCare applies the same coverage criteria to certain groups of similar medications. When PharmaCare approves Special Authority coverage for one medication in the group, coverage is automatically provided for all the drugs in that group. If the patient later requires another medication in the same group, no additional Special Authority request is necessary.
When a medical practitioner prescribes a medication similar to one the patient has taken before, the practitioner or pharmacist can contact the PharmaCare Help Desk to see if the drug is part of a "super category" for which the patient already has Special Authority coverage. If it is, another Special Authority request will not be required for the new medication.
Products not eligible for Special Authority coverage
Items not generally available for Special Authority coverage include drugs and medical supplies and devices listed as Examples of Items/Services that PharmaCare Does Not Cover.
Special Authorities and third-party insurers
Some third-party insurers cover a product only if PharmaCare has granted a Special Authority for the product and the Special Authority was granted before the prescription was filled (i.e., they do not provide retroactive coverage). All inquiries regarding retroactive coverage by a third-party insurer should be directed to the specific insurer, not to PharmaCare.
Prescriber (medical practitioner) and specialty Special Authority exemptions
On occasion, PharmaCare grants a Special Authority exemption to a medical practitioner or specialty.
A Special Authority exemption applied to a specific medical practitioner provides coverage for identified drugs for all the patients of that practitioner.
A Special Authority exemption applied to a specialty group of medical practitioners provides coverage for identified drugs for all the patients of all the practitioners in that specialty group.
Although PharmaCare carefully reviews requests for such exemptions, only a limited number of requests can be approved.
Assumed Special Authorities
Assumed SAs reduce the number of SA requests medical practitioners are required to submit. The resulting decrease in workload improves processing times for other requests.
For specific medications, if the medication is initially prescribed by a medical practitioner who has a Special Authority exemption, in most cases, the patient is automatically granted indefinite Special Authority (SA) approval. If so, a general practitioner or other medical practitioners will not need to submit a Special Authority request to maintain the patient's coverage.
Before issuing a prescription for medications eligible for an Assumed SA, a medical practitioner can check the patient's chart to verify that the medication was initially prescribed by a specialist. If it was, the practitioner can choose not to submit a Special Authority request.
Each criteria page indicates whether or not an SA exemption exists for a specific medication.
Pharmacy Special Authority exemptions
Some pharmacies, usually hospital pharmacies, deal with patients in specialty areas. Occasionally such a pharmacy (e.g., British Columbia Children's Hospital) is granted a Special Authority exemption.
When dispensing a particular drug that would otherwise not be a full benefit, a Pharmacy Special Authority exempts patients of that pharmacy from requiring individual Special Authority approval.
A drug dispensed under a Pharmacy Special Authority becomes a full benefit for all patients of that pharmacy, subject to PharmaCare pricing polices and the usual rules of each patient's PharmaCare plan, including any deductible requirements.
Special Authorities for exceeding maximum days' supply
Rural or remote areas:
[Amended August 24, 2016] Pharmacists can call the PharmaCare Help Desk to request Special Authority Exemptions to the PharmaCare 30-day maximum supply limit for patients residing in rural or remote areas for whom travel to the pharmacy is a significant barrier.
The exemption will be entered into PharmaNet as a one-day Special Authority.
Chronic conditions:
Medical practitioners can submit a Special Authority, requesting that a patient be exempted from the 30-day maximum supply policy for 'short-term' drugs if the patient has a chronic condition.
Short-term drugs include all narcotics, all antibiotics, antifungals, sedatives, sleeping pills, barbiturates and all medications in the Palliative Care Drug Plan (Plan P) Formulary.
Approval may be granted to allow a maximum 100-day supply.
Procedures
PROCEDURES FOR MEDICAL PRACTITIONERS:
Obtaining Special Authority for drug coverage for an individual patient
A medical practitioner submits information outlining the exceptional needs of the patient by
- Submitting a Special Authority Request form by fax, or
- By telephone, using the Practitioner Special Authority phone line
Obtaining Special Authority exemption for patients with chronic conditions
If a patient has a chronic condition requiring repeated treatment with a short-term drug, their medical practitioner can submit a Special Authority asking that the patient be exempted from the 30-day maximum supply policy for 'short-term' drugs.
If Special Authority coverage is granted, an entry is made in PharmaNet allowing a maximum 100-day supply of the specified drug.
PROCEDURES FOR PHARMACISTS:
Obtaining Special Authority exemptions for patients in rural or remote areas
[Amended August 24, 2016] Pharmacists can call the PharmaCare Help Desk to request Special Authority Exemptions to the PharmaCare 30-day maximum supply limit for patients in rural or remote areas for whom travel to the pharmacy is a significant barrier.
The exemption will be entered into PharmaNet as a one-day Special Authority.
Section 6.3 Tools and Resources
- For specific criteria for individual products, visit the Special Authority drug list or use the BC PharmaCare Formulary Search
- Pharmacists can phone the PharmaCare Help Desk to enquire about Special Authority coverage for specific patients
- Limited coverage drugs webpage
- Special Authority webpage
Section 6.4 – Collaborative Prescribing Agreements
General Policy Description
For some Limited Coverage drugs, PharmaCare invites physicians to sign a Collaborative Prescribing Agreement (CPA).
A CPA may be offered to specialists who commonly prescribe a medication for patients who meet PharmaCare coverage criteria.
Policy Details
Physicians who enter into a CPA are exempt from completing Special Authority requests for a specific drug and are subject to the terms of the CPA.
The CPA indicates the specified criteria for prescribing the drug.
If a patient does not meet the criteria set out in the CPA, the CPA requires the physician to either
- Write “Submit as zero cost to PharmaCare”/”PharmaCare pays zero” on prescriptions, or
- Submit a Special Authority request for exceptional coverage
Pharmacists filling prescriptions marked as "Submit as zero cost to PharmaCare"/"PharmaCare pays zero" must enter the intervention code DE Adjudicate to $0.00 as requested to ensure PharmaCare does not cover the cost.
PROCEDURES FOR PHARMACISTS:
Processing Prescriptions that state "Submit as zero cost to PharmaCare"
When you receive a prescription with this note, submit the claim with the intervention code DE Adjudicate to $0.00 as requested.
This ensures appropriate PharmaCare coverage and accurate prescribing feedback to physicians.
Section 6.4 Tools and Resources
- Coverage criteria, forms, and Collaborative Prescribing Agreements for Limited Coverage drugs are provided on the drug coverage criteria pages accessible from the list of Limited Coverage drugs on the Special Authority web page
- Limited coverage drugs webpage
Section 6.5 – Drug Review Process
General Policy Description
PharmaCare seeks to provide coverage for drugs that support the health and well-being of B.C. residents and that offer strong value for dollar.
Before a drug can be included in the PharmaCare formulary, it undergoes a thorough review to determine whether it meets these two requirements. The review process helps ensure the PharmaCare program remains fair, effective and sustainable.
Policy details
NATIONAL AND PROVINCIAL DRUG REVIEW PROCESS:
Drug review processes have three stages.
Stage one – Health Canada
All drugs sold in Canada must have received a Health Canada Notice of Compliance (NOC). Before issuing an NOC, Health Canada reviews the:
- Drug’s safety
- Effect of the drug when compared to taking no drug at all, and
- Quality of the manufacturing process used to make the drug
>> Learn more about Health Canada’s drug review process.
Stage two – Canada’s Drug Agency (CDA) review and recommendation
The CDA drug reimbursement review process involves a thorough and objective evaluation of the clinical, economic, patient and clinician evidence on drugs, and uses these evaluations to provide reimbursement recommendations and advice to Canada's provincial and territorial drug plans, including BC PharmaCare.
Drug submissions reviewed by CDA include new drugs introduced in Canada or new Health Canada-approved uses of existing drugs.
The review process considers
- How well the drug works when compared to similar drugs that are used to treat the same condition, and
- Whether the drug provides value for money
A team of independent experts is assembled to review each drug, and based on their findings, the committee will issue a recommendation to cover the drug, cover it if certain conditions are met, or not cover it.
>> Learn more on the CDA website.
Stage three – Ministry of Health drug review
The Ministry of Health conducts its own review before making a coverage decision. This review builds on the work done by Health Canada and CDA.
>> Read more about patented drug submission requirements.
REVIEW PROCESS IN B.C.
The review process in B.C. involves two entities: the Drug Benefit Council (DBC) and the Ministry of Health.
>> Learn more about the drug benefit council on the PharmaCare Drug Review web page.
The DBC is an independent advisory committee made up of nine professional members with expertise in critical appraisal, medicine, ethics, pharmacy and health economics, and three members from the public. Their task is to review drug submissions and make recommendations to the Ministry.
1. Drug Review Resource Committee (DRRC)
When the patented drug has gone through the necessary Health Canada and CDA reviews, the Ministry starts its review. The Ministry sends the drug submission to the DRRC, a subcommittee of the DBC.
The DRRC establishes the review requirements, including requesting reports or other inputs required for each drug submission. The DRRC also assigns expert review teams, called Drug Review Resource Teams (DRRTs), to complete the required review reports for each drug submission.
Depending on what reviews have been done to date, requested reports may include clinical evidence, clinical practice and pharmacoeconomic reviews.
2. Drug Review Resource Teams (DRRT)
Each DRRT produces written reports on their assigned drugs and forwards these to the drug sponsor for review. The drug sponsor can submit written comments for the DBC to consider in its review.
This is one of the four points at which health professionals can become engaged in the review process. See Health Industry Professionals for details.
3. Patient input through Your Voice
The Ministry invites input from patients, caregivers and patient advocacy groups through Your Voice.
4. Drug Benefit Council (DBC) review
All DRRT reports, drug sponsor written comments, patient input and other review documents are forwarded to the DBC.
The DBC reviews all the documents and makes a recommendation to the Ministry about covering the drug. The DBC recommendation includes
- Whether or not to cover the drug
- How to cover the drug (i.e., as a regular benefit for everyone or covered only under certain circumstances)
The DBC considers the following when making a recommendation:
- Available information on the clinical effect of the drug and health outcomes
- Whether it is good value for the people of B.C.
- Whether PharmaCare already covers a drug or drugs that work as well as this one
- Clinical practice and ethical implications
- Patient input
- The sponsor’s written comments on the DRRT review reports
- The CDA recommendations, when applicable
List of DBC board members: Directory of Organizations, Drug Benefit Council – current members
5. Ministry decision
In making its drug listing decision, the Ministry considers:
- The DBC’s recommendation
- PharmaCare policy for this type of drug and other programs that exist in the Ministry
- Which PharmaCare plan(s) would cover the drug
- Whether PharmaCare has the resources to cover the cost of the drug
Conflict of interest guidelines
The Ministry is committed to a fair, independent, objective, and unbiased drug review process. All those who take part in the review of a drug submission, including members of the DBC, the DRRC, and the DRRTs, are held to the highest ethical standards when conducting their activities.
For this reason, all persons involved in the drug review process must declare any relationship they, or their immediate family, have that creates—or could appear to create—a conflict of interest. The need to disclose conflict of interest information is ongoing and is the responsibility of all involved in the review process.
The Ministry's Conflict of Interest Guidelines for the Drug Benefit Review Process (PDF, 32.6KB) state that "a conflict of interest may exist whenever a Participant or an Immediate Family Member of a Participant has a direct or indirect interest or relationship, financial or otherwise, with an Entity that may affect or reasonably appear to affect the objectivity or fairness of the Participant in the Drug Benefit Review Process."
Examples of information that need to be disclosed include: payments or research funds received from a company that may benefit from the drug review decision; financial ownership in such a company, being employed by such a company; and any arrangement or relationship through which the participant could either earn or lose money because of a Ministry drug coverage decision.
Individuals who declare possible conflict of interest information are not automatically excluded from participating in the drug review process.
Whether an individual is selected to participate or not depends upon the particular drug submission under review and is determined by the DBC and/or the DRRC. To select drug reviewers, the DRRC assesses the review requirements of the particular drug submission, the expertise of the potential reviewers, and the conflict of interest information declared by the reviewers. It is up to the DRRC to select the best reviewer without conflict of interest whenever possible. As such, the DRRC may select a reviewer with an identified conflict of interest after weighing the potential benefits and risks of including the participant in the review.
>> For full details see the Ministry’s Conflict of Interest Guidelines for the Drug Benefit Review Process (PDF, 32.6KB).
Ministry drug review timelines
When a drug needs a CDA review, the Ministry starts its own review process when the CDA process is complete (i.e., on the date when the CDA issues its final recommendation).
All other patented drug submissions start on the date the complete submission is received by the Ministry.
The Ministry's target timeline to a decision is defined as the time from when the Ministry begins its review to the time it publicly communicates its decision and usually includes completing all implementation steps.
The target timeline to a decision for a standard review is 9 months. The timeline for a complex review is 12 months. A complex review usually includes extra requirements, such as having to develop clinical coverage criteria, develop a Special Authority form, complete discussions with a pharmaceutical manufacturer, and/or complete other implementation steps as required.
Priority reviews
A drug submission may be given priority status if it
- Was granted priority review status by CDA, or
- Meets a significant clinical need and shows major therapeutic benefit, or
- Shows substantial economic benefit
Priority drug reviews will be completed within 6 months for standard submissions or 9 months for complex submissions.
Sponsor engagement in the drug review process
The Ministry provides the sponsor with the four points of engagement during the drug review process:
A. Pre-DBC: when the Drug Review Resource Team (DRRT) reports are ready for comment
The sponsor may review the reports of the Drug Review Resource Teams (DRRT) and submit written DRRT report comments to the Ministry within ten (10) business days of receiving the reports. These comments will be included in the documentation forwarded to the DBC for review. The comments should
- Indicate whether there is agreement or disagreement with the reviewers report;
- Be evidence-based and referenced, citing material from the original drug submission; and
- Not introduce new clinical evidence
New clinical evidence included in manufacturer comments will not be considered by the DBC. If the manufacturer would like new clinical evidence considered by the DBC, the manufacturer must resubmit the drug submission to CDA or to the Ministry for a non-CDA submission.
B. Post-DBC: when the embargoed DBC recommendation and reasons for recommendation are ready for review
The sharing of the embargoed DBC Recommendation and Reasons for Recommendation is intended to improve the dialogue between the Ministry and the sponsor. The sponsor will be provided with an embargoed copy of the DBC Recommendation & Reasons for Recommendation after the DBC meeting subject to requirements of a confidentiality agreement.
At the time the embargoed DBC recommendation is released, the sponsor may file a Request for Reconsideration based on grounds that either
- The Ministry and/or the DBC did not follow the proper process; or
- The DBC recommendation is not supported by the evidence or input reviewed.
This written request, directed to the Director of Formulary Management, must be filed within five (5) business days of receiving the embargoed copy of the DBC Recommendation and Reasons for Recommendation.
The Request for Reconsideration will be composed of the reason and grounds for the request, the relief sought, and supporting evidence. A Request for Reconsideration cannot be made solely because the sponsor disagrees with the recommendation. The request must identify the aspect(s) of the DBC recommendation with which the sponsor disagrees.
No new information will be considered in the reconsideration as new information requires a resubmission.
Requests are examined by the Ministry DBC Secretariat in consultation with the DBC Chair to determine whether the issue(s) raised can be resolved in discussions with the sponsor. If the Ministry is unable to address the issues, the request will be forwarded to the DBC for reconsideration.
If the Ministry does not receive a request for reconsideration after five (5) business days, the embargoed Recommendation and Reasons for Recommendation will become final.
C. Pre-Ministry of Health decision: once the DBC recommendation becomes final but prior to implementation
Once a DBC Recommendation and Reasons for Recommendation document is made final, a sponsor may engage the Ministry within 10 business days by submitting a written statement to pharma@gov.bc.ca requesting a meeting with the Ministry before the listing decision is made and implemented.
The Ministry, at its discretion, may also initiate discussions with the sponsor.
If and when discussions are initiated, the target timeline to complete discussions is 25 business days from the day the final DBC recommendation is released. The target timeline may be adjusted upon mutual agreement.
D. Post-Ministry of Health decision: after the Ministry has implemented the decision
The sponsor may file a resubmission if new information becomes available that addresses the reasons for the decision. The resubmission should be made to CDA for CDA drug submissions.
For non-CDA drug submissions, the Ministry has the discretion to determine whether the drug review reports will be made public (i.e., posted on the Ministry website) once a listing decision has been made. Prior to posting publicly, the submission sponsor will have 15 business days to review the final reports to request the non-disclosure of any specific portions that it deems to be of a confidential or proprietary nature. The timeframe may be adjusted on mutual agreement.
If the submission sponsor requests the confidential information be deleted, the Ministry will remove the confidential information by using “blacking out” redaction techniques.
Patient engagement in the drug review process
Through Your Voice, patients, caregivers, and patient groups can have input to the PharmaCare drug review process (PDF, 666KB).
Who can give input?
B.C. residents who can answer "yes" to any of the following questions for a drug on the Your Voice web page can give input:
- Do you have the medical condition for which the drug would be used?
- Are you a caregiver to someone who has that medical condition?
- Does your patient group represent patients who have that medical condition AND have you registered with PharmaCare to give input?
Drug submission requirements
Patented drug products
Drug manufacturers must apply to the Ministry of Health to have their product considered as a PharmaCare benefit.
The Ministry bases PharmaCare coverage decisions on PharmaCare policies, programs, therapeutic options, resources, and the evidence-informed recommendations of the Drug Benefit Council (DBC). The DBC's advice to the Ministry is based upon a review of many considerations, including available clinical and pharmacoeconomic evidence, clinical practice, patient and ethical considerations, and the recommendations of CDA, when applicable.
To be considered for coverage, patented product submissions for new drugs, new combination products, and drugs with new indications must first undergo a review by Canada's Drug Agency (CDA).
All other submissions, including biosimilars, line extensions and modification of criteria, should be submitted directly to the Ministry.
For more information, visit Drug reviews – information for health industry professionals.
General products
For information, refer to the Section 5.6 to 5.12 of this manual.
Review timelines
The review timeline starts when the Ministry begins its review and ends when the Ministry publicly communicates its decision.
The target timeline for a decision is 9 months for a standard review and 12 months for a complex review. A review is considered complex if clinical coverage criteria, a Special Authority request form, a Product Listing Agreement (PLA), or other similar elements need to be developed.
If CDA conducts a review, the Ministry’s review generally follows an internal 9-month timeline. For non-CDA drug submissions, the Ministry’s review generally follows a 12-month timeline.
For submissions reviewed by CDA, the start date will be the issue date of the final Canadian Drug Expert Committee (CDEC) recommendation.
For other submissions, including line extensions, coverage status and blood glucose test strips, the review start date is the date the complete submission is received by the Ministry.
Refer to the Quarterly Report on Completed Drug Submission Reviews (PDF, 514KB) for information on review periods for recently reviewed drugs.
Procedures - submitting the documents
PharmaCare accepts submissions through Dropbox or email. Review the relevant submission checklist to ensure you include everything required. Incomplete submissions will not be reviewed.
Dropbox
Please email us to request access to your secure Dropbox: DrugReviewProcess@gov.bc.ca
- Compress: Remove non-essential graphics, and compress the submission, preferably using Microsoft Windows
- Rename: Rename the compressed file to remove the .zip extension. Government email programs reject emails with a .zip attachment
- Encrypt: Encrypt the submission package using AES-128 encryption standards or better, preferably using Microsoft Windows
- Submit: Send the compressed, encrypted file to DrugReviewProcess@gov.bc.ca. Send the decryption password to the same address in a separate email. Drug Review staff will confirm they have received the submission
To request an alternative submission method, please email DrugReviewProcess@gov.bc.ca
Foreign-authorized drug submissions
PharmaCare may decide to temporarily cover an alternative drug when a benefit is in short supply. Sometimes the alternative is a foreign-labelled product. Refer to Foreign-authorized drug submission for details on submitting foreign products.
For information on drug shortages, refer to section 5.24 of this manual.
Section 6.5 Tools and Resources

7 - Coverage Plans
Section 7.1 – Plans Overview
Section 7 provides information on the 12 PharmaCare plans:
General Policies
Acute care and extended care patients
PharmaCare coverage is not extended to patients in acute or extended care hospitals unless otherwise noted under the specific plan.
Overpayment of assistance
PharmaCare may seek recovery of any overpayment of assistance if the beneficiary is later determined to have been ineligible for that assistance. This policy applies to all PharmaCare plans and to all items that PharmaCare covers.
Relationship to Other Drug Plans
Federal insurers
PharmaCare is the insurer of last resort for individuals covered by federal drug plans including the Canadian Forces, Veterans’ Affairs or Non-Insured Health Benefits.
For individuals covered under federal plans, PharmaCare covers only items eligible for PharmaCare coverage that are not eligible for coverage under the individual’s federal drug plan.
Supplemental insurers
PharmaCare does cover individuals who have supplemental health plans (usually private insurers). These insurers may pay costs not covered by PharmaCare (such as amounts under the Fair PharmaCare deductible).
PharmaCare does not coordinate or direct supplemental health plans as to who or what the private insurers cover.
>> For more information please see Section 3.4—Claims, Patients–Other Insurers.
BC PharmaCare and patient support programs
As a public support program, PharmaCare coverage is not tied to participation in a patient support program run by a drug manufacturer. Beneficiaries are not required to enrol in a private patient support program to be eligible for PharmaCare coverage.
For one in 10 prescription drugs sold in Canada, the drug manufacturer offers a patient support program. The programs offer some supports, such as patient education, for using a particular brand of drug. To enrol in a patient support program, patients are usually required to provide persona information, including medical information, which the company will share with others. PharmaCare coverage is not affected if a beneficiary voluntarily chooses to participate in a patient support program.
Section 7.2 – Fair PharmaCare
General Policy Description
BC's Fair PharmaCare plan provides B.C. families with coverage for eligible prescription drugs and designated medical supplies, based on their net income.
Policy Details
Definitions
|
Income review process |
The process by which registrants (families) may request that their appointed level of coverage be re-assessed. To be considered for an income review, the family must have experienced a decrease in family net income of at least 10%. The new income must be verified by acceptable documentation before PharmaCare will re-assess the level of coverage. |
|---|---|
|
Beneficiary |
A person who is enrolled in a PharmaCare plan. |
|
Benefit year |
A benefit year is a calendar year or “year.” |
|
Co-payment / Co-pay |
The shared payment of eligible prescription costs between PharmaCare and the family. PharmaCare shares the cost of each prescription after the family has met their annual deductible. For families that include someone born before 1940, PharmaCare co-pays 75%. For all other families, PharmaCare pays 70%. |
|
Deductible |
Each family pays 100% of their eligible costs each year until they reach their deductible, an amount determined by their family net income. After this amount is reached, PharmaCare assists with further costs for the remainder of the benefit year. Families with very low incomes are not required to meet a deductible. |
|
Dependent child |
A dependent child is, as defined by Medical Services Plan, a B.C. resident who is the legal ward or child of the registrant or registrant's spouse and meets all the following criteria:
|
|
Family |
For Fair PharmaCare purposes, a “family” includes the registrant, their spouse (if applicable), and any dependent children who are on the same Medical Services Plan (MSP) coverage as the registrant or spouse. For simplicity, the term “family” includes individuals without spouses or dependent children. |
|
Family deductible |
The maximum amount of a family will pay on eligible drug costs during the benefit year, based on family net income. Once a family's contributions towards eligible costs reach this maximum, PharmaCare pays 100% of the family's further eligible costs for the remainder of the year. |
|
Level of coverage |
The deductible, co-payment, and family maximum assigned to a family. Each family’s level of Fair PharmaCare coverage is calculated annually based on the family’s net income as reported on the federal income tax return from two years previous. |
|
Income / Net income |
A family’s or registrant’s level of coverage is based on family net income. Net income is the amount reported on Line 23600 on the Federal Income Tax form for both registrant and spouse (if applicable) less any income from a Registered Disability Savings Plan reported on Line 12500. |
|
Income bands |
Fair PharmaCare deductibles and annual family maximums are determined by comparing the family's net income to a pre-defined table of income ranges. The same deductible and annual family maximum amounts are applied to any family whose net income is in the same income band. |
|
Interim Fair PharmaCare coverage |
Coverage is initially based on the family net income the registrant declares during Fair PharmaCare registration. Any Fair PharmaCare coverage provided is subject to expiry if a signed consent is not received—or if the net income cannot be verified—within a specified time period. Any financial assistance provided is subject to recovery if the income declared at the time of registration is lower than the income as verified by the Canada Revenue Agency. |
|
Registrant |
The person who actually registers the family for Fair PharmaCare. |
|
Spouse |
For PharmaCare purposes, a spouse is a person who is either married to or living and cohabitating in a marriage-like relationship with the registrant, and may be of the same gender as the registrant. |
|
Tax year (or relevant tax year) |
The income tax year used to calculate a family's deductible and family maximum. This is two years previous to the current benefit year. For example, Fair PharmaCare coverage for 2020 is based on income earned in 2018. |
Understanding Fair PharmaCare coverage
Fair PharmaCare coverage is income-based and has three elements: the deductible, the co-payment, and the family maximum.
PharmaCare bases the coverage level for a family on their net income (verified by the Canada Revenue Agency) by consulting the income range tables listed below:
- Regular Fair PharmaCare Coverage (PDF, 185 KB)
- Fair PharmaCare Enhanced Coverage for those born in 1939 or earlier (PDF, 183 KB)
Annual deductible
Families pay 100% of their eligible prescription drug costs until they reach their annual deductible, at which time PharmaCare begins assisting them with eligible costs.
PharmaCare first determines into which income band a family’s net income falls. All families within an income band are assigned the same deductible.
The deductible may be zero for lower-income families.
Co-payment
After a family meets their annual deductible, the cost of eligible prescription drugs is shared between PharmaCare and the family for the rest of the year. For families that include someone born before 1940, PharmaCare pays 75% (the family pays 25%); for all other families, PharmaCare pays 70% (the family pays 30%) until the family meets their family maximum.
Family maximum
The family maximum is the most a family will pay towards eligible drug costs in a year. Costs the family incurs during the year before meeting their deductible, and all the co-payments they make after meeting their deductible, count toward their family maximum.
A family’s maximum is determined in the same manner as the annual deductible but the percentage used is higher.
Once a family meets their family maximum, PharmaCare covers 100% of the family’s eligible drug costs for the rest of the year.
At the beginning of each benefit year, PharmaCare re-calculates the coverage levels assigned to each family registered for Fair PharmaCare. Based on net family income from two years previous, families are assigned a new deductible and family maximum. Coverage levels change only if a family’s net income places them in a different income range.
Confirmation of coverage
Registrants can request a Confirmation of Coverage form detailing their coverage for the current year. Registrants can request the form online or contact HIBC. Registrants can also request this information over the phone if they provide the correct identifying information to the Income Review Unit.
>> See Release of income information.
Registration
General registration
All British Columbians with active Medical Services Plan coverage, even if they are covered under another primary PharmaCare plan (i.e., Plan B, C, or W) are encouraged to register for Fair PharmaCare.
To receive a level of coverage based on their family net income, a family must register for Fair PharmaCare and provide written consent for PharmaCare to verify their net income with the Canada Revenue Agency (CRA).
Families who do not register for Fair PharmaCare and who do not receive coverage under another PharmaCare plan are assigned the annual default deductible/family maximum of $10,000 per family member (i.e., for each Personal Health Number).
Families who register but do not consent to have their income verified by the CRA are assigned the annual default deductible/family maximum of $10,000 per family.
Individuals can register through Fair PharmaCare online registration or by contacting HIBC.
Consent form
As part of the registration process, new Fair PharmaCare registrants (and their spouse, if applicable) must sign and submit a consent form authorizing PharmaCare to verify their income with the CRA.
Temporary coverage
From the date a family registers in Fair PharmaCare to the date that PharmaCare receives CRA verification of a family’s income, PharmaCare assigns temporary coverage for the family based on the income declared during their registration for Fair PharmaCare.
If PharmaCare does not receive a signed consent form from the registrant (and spouse, if applicable) within 60 days of the family’s initial registration, temporary Fair PharmaCare coverage may expire, and the family will be assigned the default annual deductible/family maximum of $10,000 per family.
After the signed consent form is returned and processed, PharmaCare assigns 30 days’ additional temporary coverage during which time PharmaCare verifies the family’s income information with the CRA.
When CRA verifies the family’s income information, PharmaCare establishes ongoing coverage based on the verified family’s net income and mails the family a Confirmation of Coverage letter.
If the family information provided during registration does not match CRA data (e.g., the Social Insurance Numbers or birth dates do not match or CRA finds no record of a tax return for the applicable year), PharmaCare provides 60 days’ additional coverage to allow the family time to correct the erroneous information or file a return.
If, after this period, the registrant has not filed an income tax return for the requested year, Fair PharmaCare coverage is set to the default annual deductible/family maximum of $10,000 per family; the family must file an income tax return for the applicable year and send a copy of their resulting CRA Notice of Assessment in order to have their coverage based on their actual family net income.
Coverage start date
Coverage begins immediately for those who register by phone or online. If a person registers using a paper registration form, coverage starts the day the registration is processed and approved by HIBC.
The start date of Fair PharmaCare coverage depends on an individual’s MSP coverage, the date the individual registered, and whether PharmaCare is able to verify income as described in the table below:
| Individual is... | Coverage |
|---|---|
| Not enrolled in MSP and not registered for Fair PharmaCare | Eligible prescription and medical supply costs do not count towards the Fair PharmaCare deductible and family maximum, nor can those costs be reimbursed. |
| Enrolled in MSP but not registered for Fair PharmaCare | Eligible costs for the current benefit year (from the later of January 1 or the date the individual or family enrolled in MSP) count towards the Fair PharmaCare deductible and family maximum but cannot be reimbursed. |
| Enrolled in MSP and registered for Fair PharmaCare | Eligible costs for the current benefit year count towards the Fair PharmaCare deductible and family maximum (from the later of the date the individual enrolled in MSP or the date the individual registered for Fair PharmaCare). After the individual or family meets their deductible, PharmaCare contributes to their additional eligible costs for the rest of the year. |
Individuals will not be reimbursed for costs from the following:
- Previous benefit years, regardless of when they registered for Fair PharmaCare
- The current benefit year before the date they registered for the plan
- The current benefit year if PharmaCare is not able to verify their family net income before December 31, or
- Periods during which their MSP coverage was not active
Eligibility
Basic eligibility
To be eligible for coverage under Fair PharmaCare, a person must have the following:
- Active Medical Services Plan (MSP) coverage
- A Social Insurance Number (SIN) assigned by the Government of Canada
- Filed an income tax return for the relevant taxation year (that is, two years ago) or, if they were not a resident in Canada at that time and could not therefore file a tax return, provide sufficient documentation of their world income (see New Canadian residents)
When a person enrols in MSP, they receive a BC Services Card with a lifetime Personal Health Number (PHN) that gives them access to publicly funded health services in British Columbia.
If a registrant or spouse does not meet the eligibility requirements, the eligible members of their family can still register.
If a registrant or their spouse does not have a Personal Health Number (BC Services Card number), they must register by phone.
Specific eligibility issues
Individuals covered by other PharmaCare plans
These five primary plans cover a wide range of benefits and may be combined with a specialty plan:
- Plan B (Long-term Care)
- Plan C (Income Assistance)
- Plan F (At-Home Children)
- Plan W (First Nations Health Benefits)
- Fair PharmaCare
Note: Individuals covered by Plan C and F are encouraged to register for Fair PharmaCare in advance. In this way, if coverage through Plan C or Plan F ends, coverage through Fair PharmaCare will begin automatically.
These seven supplemental plans cover specific items related to specific conditions and patient groups:
- Plan D (Cystic Fibrosis)
- Plan G (Psychiatric Medications)
- Plan P (Palliative Care)
- Plan M (Medication Management)
- Plan S (Smoking Cessation)
- Plan X (British Columbia Centre for Excellence in HIV/AIDS)
- Plan Z (Assurance)
- For coverage of prescription medications and medical supplies not covered under the supplemental plans, individuals must register for Fair PharmaCare or qualify for coverage under another primary plan.
Spouse covered under a federal drug plan
Spouses eligible for drug coverage from a federal insurer (i.e., Non-Insured Health Benefits through Health Canada, Veterans Affairs Canada, or the Canadian Forces) are still considered a member of the Fair PharmaCare family. As such, their income is included in the calculation of family net income.
Drug costs covered by a federal insurer do not count toward a family’s annual deductible or family maximum.
B.C. residents studying out of province
A person who is absent from British Columbia to attend a university, college, or other educational institution retains their PharmaCare coverage if they meet the following MSP Absence for Study requirements:
- The university, college, or other educational institution is recognized by the Medical Service Commission
- The person is in attendance at that educational institution on a full-time basis
- At the time of leaving the province, the person meets MSP residency requirements
A person who is studying out of province is no longer eligible within one month of the last day of the month in which the person ceased to be in full-time attendance at the university, college, or other educational institution.
A spouse or child who accompanies a deemed resident is also deemed to be a resident if, at the time of leaving British Columbia, the spouse or child meets the MSP residency requirements.
Although an individual studying out of province may retain their PharmaCare coverage, PharmaCare will only cover drugs prescribed and dispensed in British Columbia.
Foreign students
Foreign students are generally not eligible for PharmaCare coverage as they do not meet PharmaCare eligibility requirements. The only exception is students who are employed by their B.C. educational institution and have been assigned a Social Insurance Number and who, therefore, file income tax returns in Canada. These students are eligible for PharmaCare coverage.
Foreign diplomats
Non-Canadian diplomats employed with Canadian-based commissions or foreign consulates are eligible for PharmaCare coverage. To receive PharmaCare coverage, they must
- Have active coverage under the BC Medical Services Plan, and
- Provide the following documents to PharmaCare:
- a photocopy of their diplomat card, and
- a signed and notarized affidavit, provided annually, attesting to the equivalency of the stated income
PharmaCare Coverage as a Family
General
For Fair PharmaCare registration purposes, a family includes
- The registrant
- The registrant's spouse, if any; and
- Any dependent children on the same Medical Services Plan (MSP) contract as either the registrant or the spouse.
All members of the family must be declared when registering, including non-resident spouses and federally covered spouses (whose net income is included in the family’s total net income).
Former spouses
In the case of divorce, former spouses must apply for separate Fair PharmaCare coverage.
Former spouses who are separated but not divorced may choose to remain on the same Fair PharmaCare family account if they have maintained a joint MSP contract.
Member of only one family
Under Fair PharmaCare, each member of a defined family can be registered with, and included in, only one family.
Although dependent children can be included on both parents’ MSP contracts, they can be included in only one family’s (one parent’s) Fair PharmaCare registration at any given time.
Children not on the MSP contract of either the registrant or spouse
A dependent child must be included in the registrant’s or spouse’s MSP contract to be included in a Fair PharmaCare family.
Children added to an MSP contract
A child is registered with a family upon being added to the family’s MSP contract. This ensures that the child’s drug costs will count toward the family’s deductible/family maximum.
Joint custody of children
In shared custody arrangements, only one parent may include a dependent child in their Fair PharmaCare coverage.
Dual MSP coverage
If a child is included on the MSP contract of both parents, either parent may include the child in their Fair PharmaCare registration. The parent can ask to have the child included in their Fair PharmaCare family by contacting the Fair PharmaCare Income Review Unit.
PharmaCare will include the child in the Fair PharmaCare family of the parent who first requests that the child be added.
If a parent disputes the inclusion of their child in their ex-spouse’s Fair PharmaCare family, the Income Review Unit will require the disputing parent to provide:
- A letter of agreement from the other parent to make the change, or
- Court documents indicating that they have greater than 50% custody, or
- An affidavit attesting that they have greater than 50% custody
Single MSP coverage
If a child is included on the MSP contract of only one parent, the child is included in that parent’s Fair PharmaCare family.
If the other parent wishes to include the child in their Fair PharmaCare family, the parent(s) must first contact MSP to have the child added to the correct MSP contract before contacting the Fair PharmaCare Income Review Unit to request a change in their Fair PharmaCare family record.
Family Changes
General
Fair PharmaCare registrants and their spouses are advised to notify both MSP and PharmaCare of any changes to their family structure.
Aging-out of a dependent child
When a former dependent child no longer meets the Medical Services Plan criteria for a dependent child and is therefore no longer eligible for inclusion on their parents’ MSP contract and Fair PharmaCare registration, it is the former dependent’s responsibility to register for separate coverage.
Unless they meet other aspects of the MSP criteria for a dependent, a dependent child loses eligibility for MSP under their parents’ contract on their 19th birthday. They must apply for separate MSP coverage before their birthday.
A dependent child is included in the parents’ Fair PharmaCare family until the end of the last calendar year in which he/she qualifies as a dependent child as defined by MSP.
The dependent child’s drug costs count toward the family’s deductible/family maximum until the end of that year.
The ‘aged-out’ dependent must register separately for Fair PharmaCare coverage before the end of the year in order to receive coverage based on their income.
If they do not register for separate Fair PharmaCare coverage, they are assigned the maximum deductible/family maximum of $10,000 starting January 1 of the year following the year in which they no longer qualified as a dependent child.
A former dependent child who has not filed a tax return with the CRA is permitted to provide a signed affidavit as proof of income for the first year after they no longer qualify as a dependent child (e.g., child dependents who have reached age 19, or who have turned age 25 and are no longer attending full‑time school or university). For subsequent years, they must file an income tax return.
Death of a family member
Under Fair PharmaCare, if a family member dies mid-year, the deceased family member’s accumulated drug expenditures for the year count towards the family’s deductible and family maximum and the deceased family member is automatically removed from the family’s Fair PharmaCare registration at the end of the year.
On request, a surviving spouse can request a ‘best coverage assessment’ through the Fair PharmaCare Administrative Review Unit. This assessment determines which of the following options is of greater benefit to the family:
- Removing the deceased person from the family’s PharmaCare record immediately (thereby excluding the deceased spouse’s income from the calculation for coverage and deducting their eligible expenses from the family’s accumulation towards the deductible), or
- Maintaining the current family structure until the end of the year.
If mid-year removal of a deceased individual from the Fair PharmaCare family record is beneficial to the family, the change in coverage becomes effective as of the date of death.
If the deceased spouse was born before 1940 and the surviving spouse was not, the surviving spouse will retain the coverage offered to pre-1940 families for the rest of the year, then revert to regular Fair PharmaCare coverage.
Special exceptions may permit PharmaCare coverage to continue for a surviving spouse if they previously filed their income taxes under their spouse’s tax return.
>> See Filing under a spouse’s tax return.
Income
Tax year
The income tax year used to calculate a family’s PharmaCare coverage is two years before the benefit year. For example, Fair PharmaCare coverage for 2020 would be based on income reported to the Canada Revenue Agency (CRA) for 2018.
Net income
Under Fair PharmaCare, a beneficiary’s level of coverage is based on family net income. This is the amount reported on Line 23600 on the Federal Income Tax form.
For Fair PharmaCare purposes, a family income includes the income of both the registrant and their spouse, if any. If a spouse has not filed a tax return for the relevant year, PharmaCare may refer to Line 30300 (Married Amount), line 51050 or the GST Credit Application on the beneficiary’s income tax return in order to determine the spouse’s income.
Income from a Registered Disability Savings Account, declared on Line 12500 of the Federal Income Tax form, is not included as income for the purpose of registering for Fair PharmaCare.
Alternate proof of income may be accepted in specific situations as described in the sub-sections below.
A registrant or their spouse can provide alternate proof of income if the registrant or spouse could not file a federal income tax return two years previous to the current year because that registrant or spouse was
- Not a resident of Canada, or
- A dependent child, or
- A diplomat, or the spouse of a diplomat, accredited to represent another country in Canada, or
- A member of a religious order who took a vow of poverty and whose remuneration was paid to the religious order directly or by the registrant or their spouse, or
- The Minister permits the registrant to provide alternate proof of income.
Alternate proof of income must be
- A CRA Notice of Assessment for the year previous to the current year, if available, or
- If a Notice of Assessment is not available, a notarized affidavit, signed by the person who is the subject of the affidavit and attesting to that person's net income, stated in Canadian dollars, for the year previous to the current year.
A registrant who provides proof of income for a member of a religious order who has taken a vow of poverty and whose remuneration was paid to the order directly or was paid to the order by the registrant or spouse must also provide a letter from the person's religious order confirming that all the person's remuneration was paid to the order directly or by the registrant or spouse.
In exceptional cases, PharmaCare may waive or modify the requirements above if
- Income can reasonably be determined through another form of proof,
- The registrant or a family member of the registrant would suffer undue hardship if another form of proof were not accepted, or
- It would otherwise be in the public interest to do so.
New Canadian residents
A registrant who has recently met the Canadian residency requirement and therefore did not file a Canadian tax return for the relevant tax year may still register for PharmaCare coverage. In these cases, PharmaCare accepts other proof of income:
- If the family filed a tax return for a more recent year, PharmaCare will accept a CRA Notice of Assessment for a more recent full (12-month) tax year along with a signed Income Review Application (PDF, 470KB) form.
- If the family has not filed taxes for a full tax year, PharmaCare will accept a notarized affidavit.
New residents who provide an affidavit as proof of income are eligible for an income review if their income changes after the affidavit is signed.
Spouse with federal drug coverage
The net income of a spouse with federal drug coverage (i.e., through Veterans Affairs Canada or the Canadian Forces) must be included in the family net income for the purposes of Fair PharmaCare; however, the federally covered spouse is not eligible for PharmaCare coverage for items covered by their federal plan.
Income of a non-resident spouse
The net income of a spouse who is a non-resident must be included in the family net income for the purposes of Fair PharmaCare.
If the spouse lives in a Canadian province or territory other than British Columbia, PharmaCare will confirm the spouse’s income through the CRA.
If the spouse does not live in Canada, PharmaCare requires a notarized affidavit—which must reflect one full year of income stated in Canadian dollars—as documentation of foreign income.
Filing under a spouse's tax return
The CRA allows the spouse of a person who earned less than a determined amount (e.g., less than $10,527 for the 2018 tax year) to claim the spousal amount on Line 30300 (“Spouse or Common Law Partner Amount”) of their spouse’s tax return, rather than filing their own tax return.
If the spouse who files taxes dies, PharmaCare coverage for the surviving spouse continues for the rest of the year.
In the following year, coverage continues uninterrupted if PharmaCare is able to verify the surviving spouse's income with the CRA and providing that:
- The family’s registration status remains the same (i.e., the deceased spouse was not de-registered from Fair PharmaCare), and
- The family’s taxes are filed and processed for the relevant year (e.g., filed 2018 taxes for 2020 benefit year).
If PharmaCare cannot verify the surviving spouse's income with the CRA (for example, if the deceased spouse was de-registered from Fair PharmaCare so that the deceased spouse’s income would not be included when calculating the surviving spouse’s level of Fair PharmaCare coverage), PharmaCare will contact the surviving spouse to ask them to either file a tax return or provide documented proof of income. Cases are handled in the same manner as a Fair PharmaCare Income Review.
This policy also applies to any individual who has declared their income on Line 30300 of their spouse’s income tax return but who is noted in the CRA data as someone who “did not file” (as may happen in the case of divorce, separation, or an error in filing or processing a tax return).
Spouse in a long-term care facility
The net income of both spouses is automatically included in a family’s net income amount. However, if a family experiences financial difficulty because one spouse is moved to a licensed long-term care facility (where they are covered under PharmaCare Plan B), the family can request an income review.
The request for an income review must be made before the end of the benefit year for which reassessment is needed.
In this situation, as it is under Medical Services Plan policy, the spouses’ MSP coverage must be separated into two individual contracts. This allows each spouse to be a separate family under Fair PharmaCare and for the level of coverage of the spouse living at home to be based on their net income only.
If the couple does not meet the MSP criteria for separate contracts, but their combined net income is less than $42,000 (or the care facility cost reduces net income to less than $42,000), PharmaCare will consider removing the long-term care spouse from the Fair PharmaCare family record if the situation constitutes financial hardship for the spouse not in long-term care.
CRA income reassessment
The CRA can reassess an income return up to four years after sending the original Notice of Assessment. If a reassessment results in a lower net income that would place a person in a lower Fair PharmaCare income range (i.e., a higher level of coverage), it is the person’s responsibility to inform PharmaCare of the change and ask for an Income Review.
Members of religious orders who take a vow of poverty
If a B.C. resident who has taken a vow of perpetual poverty as a member of a religious order earns an income, the CRA requires them to file a tax return. When registering for Fair PharmaCare, these individuals can deduct the income or pension earnings they have donated to their order from their net income as reported on their tax return.
If a person has taken a vow of poverty, does not earn an income, and does not file income taxes, PharmaCare requires them to submit an affidavit attesting to this before it can provide Fair PharmaCare coverage. On the affidavit, the person must declare their entire income—including any amounts donated to the religious order.
After the person registers, PharmaCare will ask them to submit a signed consent form allowing PharmaCare to verify their income with the CRA in future years. If the person should begin earning an income, this ensures uninterrupted coverage.
If PharmaCare is unable to verify a registrant’s family net income, PharmaCare cannot determine the appropriate level of Fair PharmaCare coverage for the family:
- Families that do not register for Fair PharmaCare are assigned an annual default deductible of $10,000 per family member (i.e., per Personal Health Number).
- If a family has registered for Fair PharmaCare, but PharmaCare cannot verify their income with the CRA (for example, because they have not filed income taxes or have not returned a signed consent form), the family is assigned an annual default deductible of $10,000 (i.e., family per registration number).
Unverifiable income
If PharmaCare is unable to verify a registrant’s family net income, PharmaCare cannot determine the appropriate level of Fair PharmaCare coverage for the family:
- Families that do not register for Fair PharmaCare are assigned an annual default deductible of $10,000 per family member (i.e., per Personal Health Number).
- If a family has registered for Fair PharmaCare, but PharmaCare cannot verify their income with the CRA (for example, because they have not filed income taxes or have not returned a signed consent form), the family is assigned an annual default deductible of $10,000 (i.e., family per registration number).
Unverifiable income (individuals age 75 and up)
Individuals who are permanently exempt from the requirement to show proof of income must be:
- 75 years or older,
- Were on MSP Premium Assistance in 2003 (the first year of Fair PharmaCare), and
- Were identified in 2003 as not having filed an income tax return
For these beneficiaries, PharmaCare assigns
- An annual deductible of $0;
- A co-pay amount of 30%; and
- An annual family maximum of $150
This policy applies only to those who were identified in 2003 as not having filed an income tax return. Those identified as not having filed in subsequent years (i.e., 2004 or later) are not exempted from the requirement to file, regardless of age.
Increased Coverage and Payment Options
General
Fair PharmaCare offers access to increased coverage through an Income Review if
- A family’s net income has decreased by 10% or more in the previous year; or
- One spouse has begun living permanently in long-term care and including that spouse’s income in the calculation of their Fair PharmaCare coverage presents a hardship for the other spouse
PharmaCare also offers a Monthly Deductible Payment Option (MDPO) for those individuals or families who have a deductible and find it difficult to pay their prescription costs early in the year.
If a family experiences a decrease of 10% or more in family net income between the relevant tax year and a more recent tax year (including the current year), the family can ask for a review of their level of Fair PharmaCare coverage. This is called an “income review.”
Individuals can apply for an income review only for the current year and must submit their application before December 31. Individuals cannot apply for a review for a previous year.
The decrease in family net income must be substantiated by acceptable documentation for the appropriate year (e.g., record of employment, copy of Employment Insurance or Canada Pension Plan payments, letter from employer, or proof of receipt of BC Income Assistance). Other proof of income may be accepted on an individual basis, upon approval by PharmaCare’s Income Review Unit.
New Canadian residents who originally confirmed their income via affidavit may also request an income review if they experience a decrease of 10% or more in family net income.
If an income review determines that a family’s net income dropped by 10% or more, and the change in income warrants a change in the level of coverage, PharmaCare lowers the family’s annual deductible and family maximum accordingly and mails them a new Confirmation of Coverage.
>> For information on the effective date on which a family’s coverage changes as a result of an income review, refer to Payment Reconciliation–Retroactive Reimbursement and PharmaCare Recovery.
As a result of a change in a family’s level of coverage, a registrant may be eligible for reimbursement of expenses incurred earlier in the year that exceed the new family deductible and/or maximum. If the reimbursement owing is more than 2% of a family’s net income, PharmaCare issues payment immediately if the registrant makes a written request. If the retroactive payment is less than 2% of family net income, or if the family does not make a written request for immediate payment, the payment is issued after the end of the year.
Eligibility for an income review and any change in a family’s level of coverage may be revoked if CRA does not later verify the net income on which the income review was based. If, after a change in a family’s level of coverage, the CRA’s subsequent income tax assessment does not support the claim of reduced income, PharmaCare will recover any overpayment to the registrant.
Monthly Deductible Payment Option (MDPO)
The MDPO allows families to spread their Fair PharmaCare deductible over the course of the year. Once enrolled, families pay their Fair PharmaCare deductible in monthly instalments and receive PharmaCare assistance with eligible prescription and medical supply costs right away.
Eligibility
Families meeting the following criteria are eligible to enrol in MDPO:
- Are registered for Fair PharmaCare
- Do not have private health insurance with a drug benefit plan
- Have a deductible greater than $0
- Have not voluntarily or involuntarily terminated an MDPO account and, following reconciliation, had an outstanding debt that has gone to collections
- Are current MDPO account holders who are not 90 days or more in arrears
Enrolment
Families may enrol in MDPO for the current year at any time up to the last business day of September (after which PharmaCare can accept enrolments only for the following benefit year).
Participation in MDPO expires at the end of the benefit year (i.e., December 31).
Families who want to continue participating in the MDPO must re-enrol for the following year.
Enrolment process
On request, PharmaCare provides an MDPO Enrolment Package to eligible Fair PharmaCare registrants.
On receipt of completed MDPO enrolment form(s), PharmaCare
- Verifies that the registrant is eligible,
- Opens an MDPO account for the registrant, and
- Advises Revenue Services BC (RSBC) of the person’s enrolment.
RSBC mails out a Welcome Letter explaining the option.
Re-enrolment
Families enrolled in MDPO are notified (normally in November) that they will need to re-enrol before the end of the current benefit year if they wish their enrolment to begin January 1 of the following year.
A separate MDPO account is opened for each benefit year.
Debit & credit balances
PharmaCare notifies registrants of any debit balances incurred—and the deadline for settling the balance—in January of the year following the year in which the debit was incurred.
If an MDPO account holder has incurred a debit balance of $10 dollars or more, and has re-enrolled for the following benefit year, they have until March 1 of the year after the debit was incurred to settle the debit.
If a registrant still has a debit balance after March 1, the balance from the previous year is sent for collection and the current year’s account is terminated and reconciled, with either an invoice or refund cheque issued.
Families owing less than $10 dollars will not be terminated from the MDPO.
After the end of the calendar year, or upon cancellation of enrolment in the program, an enrolee’s actual prescription costs and payments towards their deductible are reconciled. If the enrolee’s actual prescription costs did not exceed their deductible, the difference between the payments towards the deductible and the actual prescription costs is refunded to the enrolee.
Reconciliation
No debit or credit balance from a previous year will be applied to the following year’s deductible. Following account reconciliation, either a refund cheque or invoice will be issued.
Re-enrolment process
Each November, PharmaCare mails an “MDPO Re-enrolment Package” to all active MDPO account holders, with the exception of account holders whose accounts are 90 days or more in arrears at time of mail-out.
Upon receipt of completed MDPO re-enrolment form(s), PharmaCare
- Verifies that the applicant’s current MDPO account is not 90 days or more in arrears.
- If the account is 89 days or less in arrears, creates a new MDPO account; and
- Communicates the enrolment to RSBC.
RSBC mails out “Welcome Letter” to all individuals re-enrolled in MDPO
Arrears
For the purpose of the Fair PharmaCare MDPO, an "account in arrears" is defined as an MDPO account that has an outstanding debt equivalent to the sum of at least three (3) monthly payments excluding Non-Sufficient Funds (NSF) charges.
All MDPO account holders who miss one (1) payment receive the following warning in their monthly statement:
ATTENTION: Your expected payment has not been received. Please pay the “Total Amount Due” shown on the Payment Remittance Advice below or you may be required to pay the full cost of any future prescriptions.
Termination/transmission of actual financial obligations
If an MDPO account is 120 or more days in arrears, PharmaCare terminates the registrant’s MDPO enrolment and an actual financial obligation (AFO) is transmitted to RSBC.
Collection
Accounts in arrears are subject to collection action by the Province.
Tax receipts
Tax receipts are mailed to all MDPO enrolees following the close of the benefit year. This allows individuals to claim the expense as a medical expense for tax purposes.
Relationship to PharmaCare retroactive reimbursements
Each spring, PharmaCare issues cheques to families owed a Fair PharmaCare reimbursement. If a family has an outstanding debit under the MDPO, any refunds due to the family are held and applied to the outstanding MDPO debt, resulting in either a refund or the MDPO account being placed on the Collections Report.
Default Coverage/Coverage Termination/Reinstatement
General
Fair PharmaCare coverage will default to a deductible/family maximum of $10,000 per family if:
- A registrant or their spouse does not return a consent form authorizing PharmaCare to verify their income with the Canada Revenue Agency;
- A registrant or spouse withdraws their consent authorizing PharmaCare to verify their income with the Canada Revenue Agency;
- Family net income could not be verified for another reason (example.g., a registrant or spouse has not filed an income tax return).
Fair PharmaCare coverage may be terminated if:
- The registrant leaves B.C. to live elsewhere, or
- The registrant opts out of the B.C. Medical Services Plan.
Reinstating coverage
In certain circumstances, a family’s income-based Fair PharmaCare coverage may default to the $10,000 deductible/family maximum or be terminated. If the registrant becomes eligible for coverage again, they can ask to have their coverage reinstated.
Coverage can be reinstated only if the registrant, and if applicable, their spouse, takes the steps needed to meet the eligibility requirements (e.g. filing a tax return, re-enrolling with the B.C. Medical Services Plan or providing their consent for PharmaCare to verify their income with the Canada Revenue Agency).
To reinstate coverage, individuals must contact Health Insurance BC.
Payment Reconciliation–Retroactive Reimbursement and PharmaCare Recovery
Retroactivity
A family may be entitled to reimbursement for eligible expenses incurred in excess of their family deductible and/or family maximum if an income review, late registration, late filing of income taxes, or a change in family structure results in the family being assigned a new lower family deductible and family maximum during the course of the benefit year.
Retroactive payments are calculated and issued automatically in the spring of the year following the benefit year. Individuals do not need to apply for reimbursement.
To receive retroactive reimbursement for a given year, a family must have
- Registered before the end of the year (December 31),
- Had “permanent coverage” as of December 31 of the year, and
- Incurred eligible costs during the year in excess of their annual deductible and/or family maximum.
Only eligible drug and medical supply costs incurred between the “Retro Eligibility Date” (inclusive) and the “Retro Calculation Period End Date” (inclusive) are eligible for reimbursement.
Definition of "Retro Eligibility Date"
The “Retro Eligibility Date” is
- January 1, or
- The date the family registered for Fair PharmaCare, or
- The date of the most recent addition or removal of a spouse from the family Fair PharmaCare record, or
- The date the family’s income-based Fair PharmaCare coverage was reinstated after cancellation.
Definition of "Retro Calculation Period End Date"
The “Retro Calculation Period End Date” varies depending on whether the reimbursement is the result of the regular annual retroactive reimbursement calculation or the result of a request from a registrant for earlier reimbursement.
If the reimbursement is the result of the regular annual retroactive reimbursement calculation, the Retro Calculation Period End Date will be December 31.
If the reimbursement is the result of request for an “Early” retroactive reimbursement, the Retro Calculation Period End Date is
- The date the retroactive reimbursement is calculated, or
- December 31 of the previous year, if the retroactive reimbursement is calculated during the subsequent year.
Eligible costs incurred between January 1 of the benefit year and the Retro Eligibility Date count towards the deductible and family maximum for families who register later in the year, provided the family incurred the costs while actively enrolled in the Medical Services Plan (MSP). These costs, however, are not eligible for reimbursement.
Income review
Families that experience a decrease in family income of at least 10% between the relevant tax year and a more recent tax year (including the current benefit year) may request a review of their assigned deductible and family maximum. If the income review results in the assignment of a new, lower family deductible and maximum, families may be eligible for retroactive reimbursement of expenses incurred before the new deductible and maximum were assigned. The Retro Eligibility Date for these families depends on individual family circumstances:
- The Retro Eligibility Date is January 1 for families that have
- permanent eligibility status as of January 1 of the benefit year, or
- interim coverage as of January 1 of the benefit year and permanent coverage as of December 31 of the benefit year (see Late Filing of Income Taxes below)
- The Retro Eligibility Date is the date the family registered for Fair PharmaCare (see below) for families who registered with Fair PharmaCare after January 1.
- The Retro Eligibility Date is the Family Change Date (see below) for families who experienced a change in income due to a change in family structure.
- If an individual becomes eligible for retroactive reimbursement as a result of an Income Review and the amount owed to the individual is greater than 2% of family net income, the payment will be issued immediately if the individual writes a letter to PharmaCare requesting immediate reimbursement.
Late registration
Fair PharmaCare families who register partway through a benefit year are assigned interim coverage until their income can be verified by CRA. If CRA income verification results in PharmaCare assigning a family deductible and/or family maximum that is lower than the family’s interim coverage level, the family may be eligible for retroactive reimbursement at the end of the year. The Retro Eligibility Date for late registrants is the date the family registered for Fair PharmaCare.
Late filing of income taxes
For the first year in which family income cannot be verified during the Annual renewal process because a family has not filed income taxes for the relevant year, the family is assigned 60-day interim coverage, based on the previous year’s income. Interim coverage begins January 1 and expires on February 28 if income is not verified by that date. When interim coverage expires, the family is assigned the default deductible of $10,000.
>> For more information about annual recalculation of coverage, see the Annual renewal section.
In the second year in which family income cannot be verified during Annual Renewal (that is, the family has not filed taxes for two consecutive years), the family is assigned the default deductible of $10,000 effective January 1. That is, no interim coverage is provided.
If a family subsequently files taxes and PharmaCare is able to verify their family income, a new deductible and family maximum is assigned based on their actual family net income. The family may be eligible for reimbursement for purchases made between January 1 of the benefit year and the date their income was verified. The Retro Eligibility Date for registrants whose income verification was delayed due to late filing of their income taxes is January 1 of the benefit year.
Change in family structure
The addition or removal of a spouse from the registrant’s Fair PharmaCare record may change the family’s level of coverage. The Retro Eligibility Date for a change in family structure is the Family Change Date (see below). While all eligible costs from January 1 (or the registration date for late registrants) accumulate towards the deductible and family maximum, purchases made before the Family Change Date are not eligible for reimbursement.
Family change date
If a spouse is added or removed from the Fair PharmaCare family record, the Family Change Date is the date on which the change was reported to PharmaCare.
If the spouse or registrant dies, the Family Change Date is the date of death.
The Family Change Date supersedes both a new registration date and the renewal date (January 1) as the Retro Eligibility Date.
The removal or addition of a dependent does not constitute a change in family structure for the purposes of determining the Retro Eligibility Date.
'Early' retroactive reimbursement
Families that experience a significant decrease in family income between the relevant tax year and a more recent tax year (including the current benefit year) may request a review of their assigned deductible and family maximum (see Income Review).
If an Income Review results in a retroactive amount owing to beneficiaries of greater than 2% of family net income, the payment will be issued immediately, rather than at the end of the year. The client must initiate this process by sending a written request to PharmaCare. In order for PharmaCare to consider an early retroactive payment, the family income for the relevant tax year must have been verified by the CRA or through an Income Review or Affidavit.
If a beneficiary is eligible for retroactive reimbursement (of any amount) because of an administrative error on the part of PharmaCare, the reimbursement will be sent immediately upon written request to PharmaCare.
Recovery
PharmaCare will seek recovery of any payments made to individuals in excess of the coverage to which the family is entitled based on their CRA-verified income.
For the period of time between registration and income verification, interim coverage is based on the income the individual/family reported during registration.
If end-of-year reconciliation reveals that a family received PharmaCare coverage greater than their CRA-verified income warrants, PharmaCare will seek to recover any overpayment from the individual or family.
PharmaCare mails requests for repayment after the end of the benefit year.
Access to Canada Revenue Agency (CRA) income information:
PharmaCare uses personal information, including Social Insurance Number and income information, only to determine each family’s level of PharmaCare coverage. The terms of the Ministry of Health Memorandum of Agreement with the CRA allows PharmaCare to access net income from Line 23600. In situations where a registrant’s spouse has not filed an income tax return, PharmaCare may also access Line 30300 (Married Amount), the GST Credit Application, and Line 12500 (Income from a Registered Disability Savings Plan). These are the amounts used to determine family net income.
As required by CRA regulations and the Freedom of Information and Protection of Privacy Act, access to CRA data is restricted to those employees in the Income Review Unit who have a security level of Operator Level 3 or above. Registration desk employees are not able to access this information. These conditions are outlined in the consent form signed by registrants and spouses at the time of registration.
Release of income information:
A Fair PharmaCare registrant may request information about their level of coverage from the Fair PharmaCare Income Review Unit. Income Review staff can provide this information via a letter sent to the registrant, or over the phone. To obtain coverage information by phone (e.g., annual family deductible), the registrant must provide the PHN, address, and postal code on their PharmaCare record. If in doubt, Income Review staff may also request additional information such as the registrant’s date of birth, prescription history, program status, family member information, PharmaCare registration number, or address history.
Withdrawing Consent
Families can withdraw their consent for PharmaCare to verify their income information with the CRA. Doing so automatically revokes their registration.
Any individual wishing to withdraw their consent must provide PharmaCare with written notice to this effect.
Fair PharmaCare coverage continues to December 31 of the year in which consent was withdrawn at which time coverage reverts to the default deductible of $10,000 per family for subsequent years.
Families may restore Fair PharmaCare coverage by re-registering and providing the necessary signed consent.
Section 7.2 Tools and Resources
See the Fair PharmaCare webpage.
Section 7.3 – Long-term Care (Plan B)
General Policy Description
PharmaCare covers the full cost of eligible prescription drugs and designated medical supplies for permanent residents of a licensed long-term care (LTC) facility that has asked PharmaCare to list it as a Plan B facility.
When a facility is added as a Plan B facility, individuals who are permanent residents of the facility are automatically covered under PharmaCare Plan B.
Policy Details
Eligibility
Individuals living permanently in a licensed long-term care facility on PharmaCare’s list of Plan B facilities are eligible for coverage under Plan B.
Coverage is provided for individuals, rather than families.
Plan B does not apply to individuals who are:
- Staying in extended care, acute care, multi-level, or assisted living facilities.
- Receiving short-term care services, including:
- respite care
- convalescent care
- residential hospice palliative care
- short-term care for other purposes determined appropriate by a health authority to meet the unique needs of the client
These short-term residents receive PharmaCare coverage under their primary PharmaCare plan (i.e., Fair PharmaCare, Plan C, Plan F or Plan W).
Contracted pharmacies
Each long-term care facility on the list of Plan B facilities is served by one contracted pharmacy.
Every month, PharmaCare pays the contracted pharmacy:
- A fixed fee ("capitation fee") for providing services to each occupied Plan B bed in the facility (see Related Services List), and
- The full cost of eligible prescription drugs and medical supplies/devices
The monthly capitation fee is paid according to the number of beds the pharmacy has serviced, regardless of the number of residents who may have occupied the bed during the month.
Coverage start date
Individuals do not need to apply for Plan B coverage.
Facilities on the list of Plan B facilities and contracted pharmacies identify an individual’s eligibility and submit the information to PharmaCare. Plan B coverage begins the day eligibility is entered in PharmaNet.
Plan B coverage cannot be provided retroactively.
What is covered
PharmaCare Plan B covers the full cost of eligible prescription drugs and medical supplies/devices up to the maximum PharmaCare recognizes.
Eligible medical supplies/devices:
- Prosthetics
- Ostomy supplies
- Insulin pumps, with a Special Authority request
Eligible pharmacy services:
- Pharmacist-administered publicly funded vaccinations
What is not covered
- Dispensing fees. These are covered by the capitation fee. (See the Related Services List for Plan B)
- Frequent dispensing. The Frequency of Dispensing Policy does not apply to long-term care patients covered under Plan B. PharmaCare’s monthly capitation fee covers all dispensing activities for eligible benefits, regardless of frequency.
- Medication reviews. PharmaCare’s monthly capitation fee covers a similar review service.
- Professional intervention fees (Special Services fee). PharmaCare’s monthly capitation fee covers professional intervention fees.
- Clinical service fees
- Palliative care medications. Individuals covered under Plan B are not eligible for the Palliative Care Plan (Plan P). Claims submitted under Plan P for an individual covered under Plan B are subject to recovery
- Routine medical supplies. These are to be provided to clients at no charge by the long-term care facility. This includes items such as needles and syringes, blood glucose test strips, and insulin pump supplies. See the Home and Community Care policy manual for full details
Coverage under other PharmaCare plans
Pharmacies must not use another PharmaCare plan to submit a PharmaCare claim for residents of Plan B facilities. Such claims are subject to audit and recovery.
There are two exceptions to this policy:
Plan W OTC items
If a Plan B facility resident is also covered under First Nations Health benefits (Plan W), they may receive coverage for over-the-counter (OTC) items on the Plan W formulary that are not covered by Plan B.
OTC claims submitted under Plan W for FNHA clients in Plan B facilities should be entered at the eligible retail cost plus an $11 dispensing fee. PharmaCare’s Frequency of Dispensing Policy applies to these Plan W–paid claims.
The intent of this exception is to allow coverage for Plan W OTC benefits that are not covered under Plan B. Pharmacies should only bill OTCs (and OTC dispensing fees) to Plan W that are not a Plan B benefit.
After submitting an OTC claim under Plan W for an FNHA client receiving care in a Plan B facility, pharmacies serving Plan B facilities must be sure to include the Plan B facility code for all subsequent (non-OTC) claims. Without the facility code, PharmaNet will continue adjudicating claims under Plan W. For details, see the PharmaCare Newsletter article Plan B pharmacies: Submitting OTC Claims for Plan W beneficiaries (PDF, 974 KB)
Continuous/flash glucose monitors
Continuous/flash glucose monitors (CGMs/FGMs) are not covered under Plan B but can be covered under the Plan B facility resident’s non-Plan B plan (i.e., Fair PharmaCare, Plan C, or Plan W—with Special Authority).
CGMs/FGMs should be entered under the alternate plan at the eligible retail cost plus an $11 dispensing fee. PharmaCare’s Actual Acquisition Cost Policy applies to all CGM/FGM claims.
Note that the intent of this exception is to allow coverage for CGMs/FGMs for Plan B residents, since CGMs/FGMs are not covered under Plan B.
After submitting a CGM/FGM claim under a Plan B facility resident’s alternate plan, be sure to include the Plan B facility code for all subsequent (non-CGM/FGM) claims. Without the facility code, PharmaNet will continue adjudicating claims under the plan under which the CGM/FGM was claimed.
Verbal prescriptions
Community pharmacies enrolled as PharmaCare providers may submit claims to PharmaCare for prescriptions received as verbal orders by clinical pharmacists or pharmacists working in ambulatory care (Health Authority pharmacists) from practitioners in LTC facilities, including prescriptions with frequent or daily dispensing. The verbal order may be received by a licensed pharmacist or licensed pharmacy technician working in the community pharmacy, or in another setting (e.g., hospital, primary care clinic, etc.), who then relays the prescription to the community pharmacy.
A verbal prescription requires a written record that includes the information listed in the College of Pharmacists’ Health Professions Act (HPA) Bylaws–Residential Care Facilities and Homes Standards of Practice (Schedule F, Part 3), Section 6.8. Under the bylaws, the written record of a verbal prescription must include:
- “The name of the practitioner and the identification number from the practitioner’s regulatory college”; and
- “The name, college identification number and signature or initial of the registrant who received the verbal prescription.”
Requirements for faxed prescriptions in the College of Pharmacists’ HPA Bylaws–Community Pharmacy Standards of Practice (Schedule F, Part 1), Section 7.1 may also apply to verbal prescriptions. If a pharmacist working in another setting (e.g., hospital, primary care clinic, etc.) receives a verbal prescription from a practitioner that must be faxed to a community pharmacy, the prescription must be faxed from the practitioner’s place of work.
Verbal prescriptions from RNs, RPNs and LPNs
As per College of Pharmacists’ HPA Bylaws–Residential Care Facilities and Homes Standards of Practice, Section 6.9, community pharmacies may also submit claims to PharmaCare for a dispense under a prescription received as a verbal order by a facility’s registered nurse, registered psychiatric nurse or licensed practical nurse, if:
- The drug does not contain a controlled drug substance,
- The registered nurse, registered psychiatric nurse or licensed practical nurse
- writes the verbal order on a practitioner’s order form or electronic equivalent, and
- transfers the written order to the pharmacy.
>> See the College of Pharmacists’ HPA Bylaws–Residential Care Facilities and Homes Standards of Practice for more about the requirements for verbal prescriptions in long-term care facilities.
>> See the College of Pharmacists’ HPA Bylaws–Community Pharmacy Standards of Practice for more about the requirements for verbal prescriptions.
Procedures
Procedural requirements for pharmacies and long-term care facilities
Many pharmacies provide services to individuals living in long-term care facilities who are covered under PharmaCare Plan B. Continuity of service is critical for these patients.
Setting up a new facility
If a pharmacy is going to provide services to a long-term care facility that will open in the near future, the facility must first contact the PharmaCare Help Desk (i.e., Information Support) to request that they be added as a Plan B facility with BC PharmaCare.
The facility must submit the following information to Information Support, allowing thirty days’ notice:
- A completed Request for PharmaCare Plan B Services to a Long-Term Care Facility form (Information Support will fax the form to the new facility when the initial request is made.)
- A copy of the facility license
- When Information Support receives these documents from the facility, they will ensure the pharmacy is enrolled in the Plan B sub-class. If the pharmacy is not enrolled in the Plan B sub-class, the pharmacy must submit a request for the sub-class using the HLTH 5433 - Provider Information Change Form (PDF, 746KB) available at Information for Pharmacies.
- When Information Support receives the completed Request for PharmaCare Plan B Services to a Long-Term Care Facility and a copy of the facility license, and confirms the pharmacy is enrolled in the Plan B sub-class, they will link the pharmacy and the facility on PharmaNet so preparations for service can begin (e.g., setting up patient profiles and preparing dosage packages) before the opening date of the facility. To ensure timely processing, we request that pharmacies confirm they are enrolled in the Plan B sub-class prior to submitting the Request for PharmaCare Plan B Services to a Long-Term Care Facility form and a copy of the license.
Terminating a pharmacy provider service
A pharmacy provider intending to terminate services to Plan B patients must give notice to Information Support. The termination date must be the last day of a month (end of day) and notice must be given no later than the last day of the month preceding the month in which service will cease. (Please note that PharmaCare cannot make capitation payments for partial months.)
Requests for another pharmacy to provide service
If an existing facility intends to enter into a contract with a different pharmacy, the new pharmacy must
- Submit a Request for PharmaCare Plan B Services to a Long-Term Care Facility form to Information Support (or provide equivalent information to Information Support by fax or e-mail), with 30 days’ notice, along with a copy of the facility license.
- Information Support will ensure the pharmacy is enrolled in the Plan B sub-class.
- When Information Support receives the completed Request for PharmaCare Plan B Services to a Long-Term Care Facility and a copy of the facility license, and confirms the pharmacy is enrolled in the Plan B sub-class, they will link the pharmacy and the facility on PharmaNet so preparations for service can begin (e.g., setting up patient profiles and preparing dosage packages) before the opening date of the facility. To ensure timely processing, we request that pharmacies confirm they are enrolled in the Plan B sub-class prior to submitting the Request for PharmaCare Plan B Services to a Long-Term Care Facility form and a copy of the license.
Long-term care (LTC) evacuations and Plan B
LTC facilities may be evacuated due to fires, floods, or other natural disasters, and residents temporarily relocated to other LTC facilities across the province.
Continuity of care is crucial during this disruption, and PharmaCare will support the work of community pharmacies to assist evacuated residents, regardless of the Plan B status of their home facility.
Definitions
|
Definitions |
|
|---|---|
|
Home facility |
The facility a person is normally residing in |
|
Home pharmacy |
The pharmacy that provides services to the home facility |
|
Receiving facility |
The facility a person is evacuated to |
|
Receiving pharmacy |
The pharmacy that provides services to the receiving facility |
Long-term care residents who are evacuated from their home facility to a receiving Plan B facility are eligible for and will receive coverage through Plan B.
The receiving pharmacy will provide services for the evacuated residents, unless there is express direction otherwise from the receiving facility to establish an alternate arrangement and appoint an additional pharmacy. Note: The additional appointed pharmacy could be the home pharmacy or another pharmacy.
If an additional appointed pharmacy is not required
The following directions apply in the absence of an alternate arrangement.
The receiving pharmacy may bill the Plan B capitation fee for each bed occupied by an evacuated resident, including if the bed is only occupied for a partial month.
The receiving pharmacy must notify Health Insurance BC (HIBC) Information Support of the:
- Number of evacuated residents
- Name of the receiving Plan B facility
- Name of each evacuated resident’s home facility (which may or may not be a Plan B facility), and
- Date that pharmacy services begin at the receiving facility
The receiving pharmacy must also notify HIBC Information Support of the last date that pharmacy services end for evacuated residents at each receiving facility they provide services to. If requested, HIBC Information Support can provide the name of the home pharmacy, if the home facility is a Plan B facility, to facilitate any transfer of prescriptions and continuity of care.
More than one pharmacy can claim a capitation fee for a bed occupied by the same person in a month if the person is a resident of more than one Plan B facility during the month because of the evacuations. If a person is not a resident in a Plan B facility for an entire month (for example, the resident is not at their home facility for the entire month of September), the pharmacy servicing the home facility cannot bill the capitation fee for that bed for that month since the bed was not occupied.
If an additional appointed pharmacy is required
If the receiving facility has a requirement for a pharmacy other than the receiving pharmacy to dispense to the evacuated residents while they are residing in the receiving facility (e.g., for operational reasons), an alternate arrangement appointing an additional pharmacy (the “additional appointed pharmacy”) must be documented by completing the Additional Appointed Pharmacy for LTC Evacuation Form.
Instructions for completing the form:
- The receiving facility must complete Section A of the form to document the assignment of responsibility to the additional appointed pharmacy.
- The additional appointed pharmacy must complete Section B of the form to provide their consent to provide services to the evacuated residents at the receiving facility and request for related Plan B claim payments to be authorized.
- Once both Sections A and B are completed, copies of the signed form must be sent by the additional appointed pharmacy to:
- HIBC Information Support
- The receiving pharmacy
- The receiving facility
- The home facility, and to the home pharmacy (if not the additional appointed pharmacy)
If as part of urgent services for evacuated residents, a Plan B facility temporarily exceeds its licensed bed capacity, the pharmacy must notify HIBC of the excess number of beds prior to submitting an invoice for capitation fees. Pharmacies will be eligible for capitation fees for temporary Plan B beds for evacuated residents for the duration of the evacuation only if notice is provided prior to receipt of the invoice.
The receiving pharmacy may need to provide emergency supplies of medications or adapt prescriptions within their scope of practice and following the guidance provided by the College of Pharmacists of BC. This may be necessary when the resident’s home facility is served by a health authority-operated pharmacy under the Hospital Act, due to differences between the Plan B formulary and health authority formularies. The receiving pharmacy may try to adapt prescriptions to Plan B benefits.
Pharmacies are asked to hold the billing for any extra costs to patients until further direction is provided by the receiving facility’s health authority.
Pharmacies are eligible for PharmaCare Clinical Services fees for adaptations for Plan B residents.
If a resident is transferred to a private long term care facility, that is neither registered as a Plan B facility nor receives health authority support, further actions may be required, including emergency temporary Plan B facility registration. In this case, please contact the PharmaCare Help Desk.
For more information, visit Patient care during states of emergency and evacuations webpage.
Changes in facility information
Certain changes in facility information require that the local Health Authority issue an amended licence to the facility. It is the facility’s responsibility to inform the pharmacy providing services.
The pharmacy must notify Information Support (using the Request for PharmaCare Plan B Services to a Long-Term Care Facility form) if a facility experiences any of the following changes:
- Change in number of eligible Plan B beds (The pharmacy must also provide a copy of the new licence indicating the change in the number of beds.)
- Change in facility name (The pharmacy must also provide a copy of the new licence indicating the new name.)
- Change in licence number (The pharmacy must also provide a copy of the new licence indicating the new licence number.)
- Change in facility type—e.g., from complex care to multi-level care (The pharmacy must also provide a copy of the new licence indicating the change in facility type.)
Note: A change in ownership does not require an amended licence.
>> Contact PharmaCare Help Desk whenever these changes occur.
Requests for facilities to exit Plan B
A facility intending to exit from Plan B and transition to another pharmacy provider submitting claims via different PharmaCare plans must give notice to the PharmaCare Help Desk. The termination date must be the last day of a month (end of day) and notice must be given no later than the last day of the month preceding the month in which service will cease. (Please note that PharmaCare cannot make capitation payments for partial months.)
Requests for facilities to re-join Plan B
If a facility previously exited from Plan B and intends to re-join Plan B at a later date, it is the facility’s responsibility to inform the pharmacy providing services. The pharmacy must notify the PharmaCare Help Desk.and:
- Submit a Request for PharmaCare Plan B Services to a Long-Term Care Facility form to Information Support (or provide equivalent information to Information Support by fax or e-mail), with 30 days notice, along with a copy of the facility licence.
- Information Support will ensure the pharmacy is enrolled in the Plan B sub-class.
- When Information Support receives the completed Request for PharmaCare Plan B Services to a Long-Term Care Facility and a copy of the facility licence, and confirms the pharmacy is enrolled in the Plan B sub-class, they will link the pharmacy and the facility on PharmaNet so preparations for service can begin (e.g., set up patient profiles, and prepare dosage packages), before the service transition date. To ensure timely processing, pharmacies should confirm they are enrolled in the Plan B sub-class before they submit the Request for PharmaCare Plan B Services to a Long-Term Care Facility form and a copy of the licence.
Section 7.3 Tools and Resources
Section 7.4 – Income Assistance (Plan C)
General Policy Description
Plan C provides drug and limited medical supply/device coverage for those receiving income assistance through the B.C. Ministry of Social Development and Poverty Reduction, and for children and youth in the care of the Ministry of Children and Family Development (including children and youth who are in care by agreement).
Policy Details
Eligibility
Recipients of assistance (Income Assistance, Disability Assistance, Hardship Assistance and Medical Services Only) registered for Plan C by the Ministry of Social Development and Poverty Reduction receive 100% coverage of eligible prescription costs.
Children and youth in care, youth in Youth Agreements, and children and youth in Extended Family Program Agreements registered for Plan C by the Ministry of Children and Family Development receive 100% coverage of eligible prescription costs.
The Ministry of Social Development and Poverty Reduction can confirm two types of PharmaCare benefits:
- Ongoing Plan C (for recipients of Income Assistance, Disability Assistance, Hardship Assistance and Medical Services Only) coverage
- Emergency Plan C assistance (maximum 48 hours).
The Ministry of Children and Family Development can confirm
- Plan C coverage for children and youth in care and youth in Youth Agreements up to when the child or youth turns 19 years of age
- Plan C coverage for children and youth in Extended Family Program Agreements for a maximum of 30 months (children and youth supported through Extended Family Program Agreements are not extended past their 19th birthday
- Emergency Plan C assistance (maximum 48 hours)
PharmaCare cannot confirm Plan C coverage.
Plan C coverage is not extended to patients in acute or extended care hospitals or to those already covered under PharmaCare Plan B (Permanent Residents of Licensed Long-term Care Facilities).
The full terms of eligibility for Plan C are described in Section 36 of the Drug Plan Regulation of the Pharmaceutical Services Act. The Ministry of Social Development and Poverty Reduction or the Ministry of Children and Family Development is responsible for determining individual eligibility for Plan C.
What is covered?
Under Plan C, PharmaCare pays 100% of eligible drug and dispensing fee costs up to the maximum cost and dispensing fee recognized by PharmaCare.
Coverage start date
The Ministry of Social Development and Poverty Reduction or the Ministry of Children and Family Development confirms eligibility and transmits Plan C coverage information in real-time to PharmaNet. Coverage is in effect from the time it is received by PharmaNet.
Plan C coverage cannot be provided retroactively.
Emergency assistance
When the Ministry of Social Development and Poverty Reduction or the Ministry of Children and Family Development grant emergency assistance, the person may qualify for temporary PharmaCare assistance for up to a maximum of 48 hours. The coverage is the same as that available under Plan C ongoing coverage.
Eligibility information is provided to PharmaCare by the Ministry of Social Development and Poverty Reduction and the Ministry of Children and Family Development. It is in effect from the time it is entered in PharmaNet.
Emergency coverage cannot be provided retroactively.
Procedures for Pharmacists
PROCESSING NEW PLAN C PATIENTS
Eligibility is entered into the Ministry of Social Development and Poverty Reduction and Ministry of Children and Family Development database. The Ministry of Social Development and Poverty Reduction gives a form to the client.
To process a new Plan C patient
- View the patient’s Ministry of Social Development and Poverty Reduction form or the child or youth’s BC Services Card to confirm the PHN.
- Check the patient's identification.
Refer to Section 9.1—Positive Identification of Patients. - Process the prescription using the PHN on the presented form or BC Services Card.
- Return the form or BC Services Card to the patient.
Plan C assistance not in place
Occasionally, the system interface with the Ministry of Social Development and Poverty Reduction fails to transmit patient information to PharmaNet and the PharmaNet transaction will not provide the expected adjudication results.
- Call the PharmaCare Help Desk. The Help Desk will contact the Ministry of Social Development and Poverty Reduction (SDPR) or the Ministry of Children and Family Development (MCFD) to have them confirm active coverage. If coverage is confirmed, the Help Desk enters short-term assistance in PharmaNet to allow for prescription processing under Plan C.
- On receiving confirmation that short-term assistance is in place, process the prescription as usual.
- If HIBC does not confirm active coverage, the client can be referred to their local SDPR or MCFD office to confirm that they are receiving support from that ministry.
Section 7.5 – Cystic Fibrosis (Plan D)
General Policy Description
Individuals registered with a provincial cystic fibrosis clinic receive coverage of eligible digestive enzymes. These individuals are also eligible for coverage of certain vitamins, nutritional supplements and hypertonic saline solutions through their primary PharmaCare plan.
Policy Details
Patient eligibility
To be eligible for Plan D, individuals must have active Medical Services Plan (MSP) coverage.
Coverage under Plan D is for individuals, rather than families.
PharmaCare does not confirm eligibility for Plan D.
An individual must be registered with one of the province’s four cystic fibrosis (CF) clinics (listed in the Tools and Resources section below) which will confirm the individual’s eligibility for Plan D and enroll them in the plan.
Only a CF clinic can request additions, changes and deletions to PharmaCare coverage under Plan D.
Plan D coverage is not available to patients in acute or extended care hospitals.
Coverage start date
CF clinics provide eligibility information to PharmaCare. Coverage is in effect from the time it is entered into PharmaNet.
Plan D coverage cannot be provided retroactively.
Product coverage
Individuals with cystic fibrosis who are registered with a provincial cystic fibrosis clinic receive the following coverage:
| Eligible costs of digestive enzymes |
100% coverage when the product is purchased at a community pharmacy and a claim is submitted on PharmaNet. |
|---|---|
| Coverage of the eligible costs of vitamins and nutritional supplements |
Coverage under the rules of the individual’s primary PharmaCare plan (i.e., Fair PharmaCare, Plan C or Plan F) when the product is purchased at a community pharmacy and a claim is submitted on PharmaNet. |
| Coverage of the eligible costs of hypertonic saline solutions |
>> See the Cystic Fibrosis Formulary for a list of products covered.
Requirements for product coverage
Items in the Cystic Fibrosis Formulary are eligible for coverage only if they are purchased at a pharmacy in the same manner as prescription drugs. This is important because the pharmacy must submit a claim on PharmaNet at the time of purchase to initiate PharmaCare reimbursement.
Product reimbursement
Digestive enzymes and hypertonic saline solutions are reimbursed up to the PharmaCare maximum price plus a dispensing fee (up to the PharmaCare maximum dispensing fee).
>> For more information, see Section 5.6—Maximum Pricing Policy.
Vitamins and nutritional supplements are reimbursed at actual acquisition cost plus a dispensing fee (up to the PharmaCare maximum dispensing fee).
>> For more information, see Section 5.7—Actual Acquisition Cost Policy.
Procedures for Pharmacies
Dispensing cystic fibrosis items
- Consult the Cystic Fibrosis Formulary to determine if the item is covered.
- Submit the claim with the correct DIN/PIN.
For liquid nutritional supplements, the dispensed quantity claimed should be the total metric quantity dispensed. For example, when submitting a claim for case of Ensure® (12 cans @ 235 ml per can) enter the total metric volume of the case (i.e., 2820 mL).
The claim will adjudicate under Plan D or the patient’s primary PharmaCare plan, as appropriate.
Section 7.5 Tools and Resources
For information on products not listed in the Cystic Fibrosis Formulary, contact the PharmaCare Help Desk at Health Insurance BC.
For more information on eligibility for Plan D, contact the nearest cystic fibrosis clinic.
- B.C. Children's Hospital
4480 Oak Street, Vancouver BC V6H 3V4
Clinic: 604-875-2345
Email: cfclinic@cw.bc.ca
- St. Paul's Hospital (Adult)
1081 Burrard Street, Vancouver BC V6Z 1Y6
Clinic: 604-806-8522
Email: cfclinic@providencehealth.bc.ca
- Victoria General Hospital (Paediatric)
1 Hospital Way, Victoria BC V8Z 6R5
Clinic: 250-727-4247
- Royal Jubilee Hospital (Adult)
Victoria Adult CF Clinic
1952 Bay Street, Royal Block 4 - RB 422, Victoria BC V8R 1J8
Clinic: 250-519-5300 local 13395
Section 7.6 – Children in the At Home Program (Plan F)
General Policy Description
The At Home Program medical benefits of the Ministry of Children and Family Development provides community-based, family-style care for severely handicapped children who would otherwise become reliant on institutional care.
Children enrolled in the program qualify for full coverage of eligible prescription drugs and eligible medical supplies/equipment under PharmaCare Plan F.
Policy Details
Eligibility
To be eligible for Plan F, individuals must have active Medical Services Plan (MSP) coverage.
Coverage is provided for individuals, rather than families.
Children enrolled in the At Home Program Medical Benefits qualify for full coverage of eligible prescription drugs and eligible medical supplies/equipment under Plan F.
Coverage under Plan F does not extend to patients in acute or extended care hospitals.
PharmaCare does not confirm Plan F eligibility. Eligibility is confirmed by the Ministry of Children and Family Development during registration for the At Home Program.
Plan F coverage ends on the last day of the month that a child turns 18 years of age.
Coverage start date
The Ministry of Children and Family Development provides eligibility information to PharmaCare. Coverage is in effect from the time it is entered into PharmaNet.
Plan F coverage cannot be provided retroactively.
What is covered
Under Plan F, PharmaCare pays 100% of eligible drug and dispensing fee costs up to the maximum cost and dispensing fee recognized by PharmaCare.
Medical supplies and equipment eligible for coverage under Plan F include:
- Prosthetics and orthotics
- Needles and syringes
- Continuous/flash glucose monitors and blood glucose test strips
- Insulin pump supplies
- Ostomy supplies
To determine the medical benefits provided directly by the At Home Program, and not by PharmaCare, visit www.gov.bc.ca/athomeprogram.
Section 7.6 Tools and Resources
Visit the Ministry of Children and Family Development website for more information on the At Home Program.
Section 7.7 – Psychiatric Medications (Plan G)
General Description
Plan G provides coverage of certain psychiatric medications to individuals for whom the cost of these medications is a serious barrier to treatment. There are 3 types of Plan G Coverage: regular, exceptional and bridge; each has a different application process.
Policy Details
Eligibility
Regular Plan G coverage is available to individuals of any age, who:
- meet the plan’s clinical and financial criteria (details below)
- are residents of B.C. with active Medical Services Plan (MSP) coverage1
Coverage is for individuals, rather than families.
Plan G provides 100% coverage of eligible drug and dispensing fee costs (up to the maximum cost and dispensing fee recognized by PharmaCare) for certain psychiatric medications to individuals for whom the cost of these medications is a serious barrier to treatment.
Plan G assistance is not available to
- individuals living in long-term care facilities that are enrolled in Plan B (Plan B already fully covers psychiatric medications)
- individuals in acute care or extended care hospitals
- individuals with employer-sponsored drug coverage benefits
- individuals whose drug costs are covered by federal insurers
Clinical eligibility
For the patient to meet clinical eligibility requirements:
- A practitioner* must assess them, and
- Prescribe them a drug covered under the Plan G formulary, and
- Certify (on the HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB)) that:
- The patient has been hospitalized for treatment related to a psychiatric condition; OR
- Without prescribed psychiatric medication, the patient is likely to be hospitalized for a psychiatric condition; OR
- Without prescribed psychiatric medication, the patient, or another person, is likely to suffer serious physical or psychological harm or economic loss
*The prescribing practitioner must have a valid practitioner college ID from a B.C. or Alberta regulatory college. Their practitioner ID must have been added to and verified in PharmaNet. This is done automatically for registrants of B.C regulatory colleges, and can be done by request for registrants of Alberta regulatory colleges.
Practitioners can contact the PharmaCare Help Desk to ask about the status of their practitioner ID in PharmaNet or request that their ID be added (for Alberta registrants).
Financial eligibility
In addition to meeting clinical criteria, applicants must sign a declaration on the HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB) that:
- The cost of prescribed psychiatric drug(s) is a barrier to treatment and that they have no other financial coverage for the drug(s); and
- They are eligible for supplemental services under MSP, i.e., have an adjusted family net income of $42,000 or less.
Applying for regular Plan G
To apply for regular Plan G coverage:
- The patient and their practitioner complete the HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB).
- The practitioner faxes the application to a mental health and substance use centre (MHSUC) or a Child and Youth Mental Health service centre (CYMH) OR the patient delivers the application in person to the relevant site.
- The local MHSUC or CYMH verifies eligibility and faxes the application to Health Insurance BC (HIBC) to register the patient. Note: PharmaCare cannot independently confirm eligibility for, nor register a patient for, regular Plan G coverage.
Regular Plan G coverage is for a set period of no more than 1 year. When this period expires, the practitioner may re-apply for another year of coverage. The local MHSUC may notify the individual at the address on their Plan G application of the expiry of their Plan G coverage and the need to see their practitioner to re-apply. If no address is provided, the individual cannot be notified.
Applying for Plan G bridge coverage – urgent after-hours situations
Plan G bridge coverage is a temporary, expedited period of coverage available in urgent after-hours situations. The following facilities can apply for bridge coverage on behalf of their clients:
- Emergency departments (EDs)
- Rapid access addiction clinics (RAACs)
- Urgent and Primary Care Centres (UPCCs)
- Correctional facilities (both provincial and federal)
- Public facilities in B.C. offering substance use withdrawal management (WDM) services through health authorities
Bridge coverage enables access to medications until a patient and their practitioner can apply for regular Plan G coverage.
To apply for Plan G bridge coverage:
- The patient and their practitioner complete the HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB).
- The practitioner faxes the application directly to HIBC. The local MHSUC or CYMH does not need to verify or process the application.
Bridge coverage is for 3 months from the date the application is processed by HIBC. To maintain Plan G coverage, patients should meet with a prescriber in their community as soon as possible (i.e., within 10 weeks) to apply for regular Plan G coverage (if needed).
Previous Plan G bridge coverage does not affect a bridge coverage application—a practitioner can apply for it again for a patient who previously had bridge coverage and is again in urgent health circumstances.
Practitioners at locations eligible for bridge coverage may also submit applications for regular Plan G coverage.
Note: A practitioner may apply for exceptional coverage and bridge coverage for the same patient at the same time with a single form, sent directly to HIBC.
Applying for exceptional coverage – for patients without MSP coverage yet
Plan G coverage may be extended to new residents of B.C. who have not yet enrolled in MSP.
To apply for exceptional coverage:
- The patient and their practitioner complete the HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB)
- The practitioner completes section C of the application and certifies that:
- They have advised the patient that exceptional Plan G coverage is for up to 6 months with no possibility of renewal or extension and that;
- The patient has either applied for MSP and is waiting to be fully enrolled, or the patient understands that they must apply for MSP without delay
Note: A practitioner may apply for exceptional coverage and bridge coverage for the same patient at the same time with a single form, sent directly to HIBC.
Individuals unwilling or unable to sign an application for Plan G coverage
If an individual is unable to sign Section A of the HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB) but is willing and able to make a verbal declaration, the practitioner (or a staff member at an MHSUC or CYMH) may sign the form for them, with the indication that they witnessed a verbal declaration. This is also permitted in situations where the practitioner and the patient are in different physical locations, e.g. a telemedicine appointment.
If an individual is unwilling to sign Section A of the Application for PharmaCare Plan G, it can be signed only by a person legally empowered to act on the individual’s behalf. This person must be one of the following:
- A member of a committee appointed under the Patients Property Act
- A person acting under power of attorney
- A representative acting under a representation agreement under the Representation Agreement Act
>> For more information, see page 2 of HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB).
Coverage start/end date
The MHSUC or CYMH provides eligibility information to PharmaCare. Coverage starts the day the information is entered in PharmaNet.
Plan G coverage cannot be provided retroactively.
Regular Plan G coverage is for up to 1 year. Bridge coverage is for 3 months, but can be provided several times to the same patient. Exceptional coverage is provided for 6 months once only.
MHSUCs and CYMHs are not permitted to automatically renew Plan G coverage.
Renewal of Plan G coverage
If an individual’s Plan G coverage is close to expiring, the MHSUC or CYMH may contact the individual to confirm their continuing need for Plan G coverage and, if appropriate, initiate a visit with a practitioner to enable renewal of coverage in time to prevent a lapse in coverage.
If the renewal process is not completed before the individual’s Plan G coverage expires, coverage terminates without any additional notification.
The renewal of Plan G coverage requires an application and eligibility assessment in the same manner as for initial coverage. The clinical and financial eligibility criteria for renewal are identical to those for initial coverage.
What is covered?
Plan G covers the medications listed in the Plan G Formulary.
Drugs in the formulary identified as "Limited Coverage" require prior Special Authority approval from PharmaCare. For these medications, an individual's practitioner must submit a Special Authority Request to PharmaCare, unless a prescriber/specialty/pharmacy exemption is in place.
Items not covered under Plan G are automatically adjudicated under the individual’s primary PharmaCare plan (e.g., Fair PharmaCare, Plan C or Plan F).
Procedures for Pharmacists
To process a new Plan G patient
- Check the patient's identification
- Patients registering for Plan G with an MHSUC or CYMH should have been instructed by staff to provide their PHN, as well as a second piece of identification that meets the College of Pharmacists of BC guidelines.
>> Refer to Section 9.1—Positive Identification of Patients. - If necessary, verify a patient’s identity, including their PHN, with the local MHSUC or CYMH staff
- Process the prescription as usual
If Plan G assistance is not in place
The PharmaNet transaction will not provide the expected adjudication results if the coverage has not been entered.
- Call the PharmaCare Help Desk.
- The Help Desk will confirm whether an Application for PharmaCare Plan G has been received but not yet entered in PharmaNet, received and returned as incomplete, or not received.
- Refer the patient to their practitioner or local MHSUC or CYMH if the Help Desk has not yet received an application form.
Section 7.7 Tools and Resources
- For more information on Plan G, contact the local MHSUC (PDF, 96KB)
- Practitioners' offices can download the HLTH 3497 - Application for PharmaCare Plan G (PDF, 896KB) or request pre‑printed copies from local MHSUCs
- See Guide to Applying for Plan G Bridge Coverage (PDF, 204KB)
- Check the Psychiatric Medications Plan (Plan G) webpage
Section 7.8 – Palliative Care (Plan P)
General Policy Description
BC Palliative Care Benefits support B.C. residents of any age who have reached the end stage of a life-threatening disease or illness and who wish to receive palliative care at home.
Eligible patients receive
- Coverage of drugs used in palliative care through PharmaCare Plan P, and
- Medical supplies and equipment through their local health authority.
>> For detailed information on medical supply and equipment benefits, refer to the Home and Community Care Policy Manual.
The purpose of Plan P is to provide patients receiving palliative care at home with access to the same drugs they would receive at no charge if they were in hospital.
Policy Details
Eligibility
Coverage is provided for individuals, rather than families.
Coverage does not extend to patients in acute care.
The benefits are available to B.C. residents with active Medical Services Plan (MSP) coverage1 who
- Are living at home (defined, for the purposes of this program, as wherever the patient is living, whether in their own home or with family or friends, or in a supportive or assisted living residence, or a hospice unit in a long-term care facility (e.g., a community hospice bed that is not covered under PharmaCare Plan B),
- Have been diagnosed with a life-threatening illness or condition,
- Have a life expectancy of up to six months, and
- Consent to the focus of care being palliative rather than treatment aimed at cure
An individual’s physician or nurse practitioner confirms a patient’s medical eligibility for palliative care benefits.
A patient’s physician or nurse practitioner must certify that the patient meets the criteria for BC Palliative Care Benefits by faxing an application to PharmaCare for drug benefits under PharmaCare Plan P (PDF, 389KB) and a copy of the application to the local health authority (for the Palliative Medical Supplies and Equipment Component of the program).
1 In some instances, PharmaCare may consider extending coverage to Canadian citizens/permanent residents who are new B.C. residents and do not yet have Medical Services Plan coverage.
Coverage start/end date
Coverage under Plan P begins as soon as Health Insurance BC (which delivers operational services for PharmaCare) processes the application and enters the information in the PharmaNet system.
BC Palliative Care Drug Benefits coverage continues for as long as the person is diagnosed as requiring palliative care.
Plan P coverage cannot be provided retroactively.
What is covered
[Updated March 17, 2017] Plan P provides 100% coverage of the eligible costs of the prescription and over-the-counter (OTC) drugs listed in the Palliative Care Drug Plan (Plan P) Formulary and dispensing fee costs (up to the maximum drug cost and dispensing fee recognized by PharmaCare).
Products not included in the Plan P formulary may be covered under the patient’s primary PharmaCare plan (i.e., Fair PharmaCare, Plan C, Plan F or Plan W).
Drugs are selected for inclusion in the Plan P formulary based on the following criteria:
- The prescription or OTC drug is prescribed for pain and symptom control; and
- The prescription or OTC drug is prescribed to improve quality of life for palliative care patients; and
- Provision of the drug to palliative care patients supports and enables them to remain at home
To receive coverage of an OTC drug in the Plan P Formulary, a prescriber must write a prescription for the drug and the pharmacy must enter the drug in the PharmaNet system, which enables PharmaCare to reimburse the eligible costs.
Note: needles and syringes for administration of injectable medications are provided by the Health Authorities as medical supplies and equipment benefits.
Products not covered under Plan P automatically adjudicate under the patient’s primary PharmaCare plan (i.e., Fair PharmaCare or Plan C)
Patients should register for the Fair PharmaCare plan if they have not already done so. Fair PharmaCare covers PharmaCare benefits not included in the Plan P Formulary.
Under specific circumstances, PharmaCare will consider a request for Special Authority coverage of a drug that is not included in the Plan P Formulary.
Payment of drug costs:
When a prescription drug or over-the-counter medication prescription for an individual registered for the BC Palliative Care Drug Plan is processed, the pharmacy enters a claim for the prescription on PharmaNet. PharmaCare will then pay the pharmacy directly for
- Drug costs up to the PharmaCare maximum price, and
- A dispensing fee (up to the maximum allowable dispensing fee)
When patients do not meet the BC Palliative Care Drug Plan eligibility criteria
For patients who do not meet the criteria for Plan P, coverage options through other government insurers (such as Veterans Affairs Canada) and private insurers can also be considered.
Please note that individuals covered by the Non-Insured Health Benefits (NIHB) Program of Health Canada or Veterans Affairs Canada (VAC) require coverage under Plan P only if a medication is not covered by NIHB or VAC.
Members of the Canadian Forces receive coverage through their employers and are, therefore, not eligible for this drug plan.
Procedures
For physicians
Submitting a registration form
Once a physician or nurse practitioner has certified that a patient meets the medical criteria, the physician or nurse practitioner completes a HLTH 349 - BC Palliative Care Benefits Registration Form (PDF, 593KB) and faxes it to both Health Insurance BC (for drug coverage) and the applicable Health Authority (for assessment for palliative medical supplies and equipment).
The Freedom of Information and Protection of Privacy Act requires that the patient or the patient’s legal representative consent to the release of personal information such as basic demographic data and diagnosis. For this reason, the patient or their legal representative must sign the registration form.
To avoid delays in patient coverage, please complete all sections of the registration form. Incomplete information will require Health Insurance BC and/or the Health Authority to return the form to obtain the missing information.
Physicians must fax the form to
- Health Insurance BC at 250-405-3587, and
- Home and Community Care office of the local Health Authority
>> See Section 7.8 Tools and Resources.
Confirming enrolment in Plan P
Coverage under Plan P begins as soon as Health Insurance BC processes the registration and enters the information in the PharmaNet system. Please allow up to 12 hours for processing.
To confirm enrolment in Plan P, a pharmacist can contact the PharmaCare Help Desk. Physicians and nurse practitioners can confirm coverage using the Palliative Care Coverage Confirmation Line as described in the Prescriber Guide (PDF, 1MB) available at Palliative Care Benefits—Information for Prescribers.
Once the registration is processed, prescriptions can be filled at any pharmacy in British Columbia.
To request Plan P coverage of a drug not included in the Plan P formulary
PharmaCare will consider a request for Special Authority Plan P coverage if a drug is needed to alleviate patient discomfort and
- The drug is not included in the Plan P formulary
- The drug is not covered under another PharmaCare plan
- There is no substitute for that drug in the formulary
- A physician or nurse practitioner must send a completed HLTH 5328 - General Special Authority Request Form (PDF, 656KB) by fax. The Special Authority Program fax number can be found on the request form. Fax is the quickest method.
- The prescriber should clearly mark “For Palliative Care Registrant” on the request form to ensure it receives priority attention.
- Adequate documentation must be included with the request. A decision on coverage may be delayed if PharmaCare needs to call the prescriber for additional information.
Section 7.8 Tools and Resources
General information for patients and caregivers
PharmaCare Plan P patient information sheet (PDF, 475KB) provides details about the plan. The sheets are available in multiple languages.
Contact a physician, nurse practitioner, the local health authority or the British Columbia Hospice Palliative Care Association.
Information on medications included in the formulary
Consult the Palliative Care Drug Plan (Plan P) Formulary or call Health Insurance BC.
For physicians and nurse practitioners
Prescriber guide, registration form, Plan P formulary, and patient information sheet:
Visit Palliative Care Benefits—Information for Prescribers.
Medical supplies and equipment component:
Information regarding medical supplies and equipment—Contact the Home and Community Care (HCC) office of your local health authority. Contact information may be obtained by calling 8-1-1 or searching “home and community care” at through HealthLinkBC.
Other palliative care information
- BC Guidelines – Palliative Care
- Palliative Care Consultation Line—Physicians throughout B.C. have access to a 24/7 toll-free phone line for palliative care consultation. The phone line is staffed by palliative care physicians who offer timely clinical advice on pain and symptom management. For this physician-to-physician palliative care consultation, call 1-877-711‑5757.
Section 7.9 – Medication Management (Plan M)
General Policy Description
Plan M covers individuals for eligible medication management services (e.g., clinical services, medication review services, and publicly funded vaccinations) provided by pharmacies.
Policy Details
Eligibility
Coverage is provided for individuals, rather than families.
Coverage under Plan M does not extend to patients in acute or extended care hospitals.
Patients covered under Plan B (Permanent Residents of Licensed Long-term Care Facilities) are not covered for Medication Review Services.
Eligibility for coverage under Plan M is established in Section 8.4—Clinical Services Fees, Section 8.9—Medication Review Services and Section 8.10—Pharmacist Administration of Drugs and Vaccines.
Coverage start date
Individuals are eligible for Plan M coverage if they meet the eligibility requirements for coverage established in Section 8.4—Clinical Services Fees, Section 8.9—Medication Review Services or Section 8.10—Pharmacist Administration of Drugs and Vaccines.
Plan M coverage cannot be provided retroactively.
What is covered?
Plan M covers the cost of clinical services, medication review services and publicly funded vaccination services provided by pharmacies up to the limits PharmaCare has established for these services.
>> For information on eligible benefits and limits, see Section 8.4—Clinical Services Fees, Section 8.9—Medication Review Services and Section 8.10—Pharmacist Administration of Drugs and Vaccines.
Procedures
For procedural information for pharmacists, refer to Section 8.4—Clinical Services Fees, Section 8.9—Medication Review Services and Section 8.10—Pharmacist Administration of Drugs and Vaccines.
Section 7.10 – Smoking Cessation Program (Plan S)
General Policy Description
Plan S covers nicotine replacement therapies for individuals eligible under the Smoking Cessation Program.
Policy Details
Eligibility
Coverage is provided for individuals, rather than families.
Coverage under Plan S does not extend to patients in acute or extended care hospitals.
Eligibility for coverage under Plan S is established in Section 5.20—Smoking Cessation Program Policy.
Eligibility for coverage under Plan S is automatically recorded in PharmaNet each benefit year (using a Special Authority record).
Coverage start date
Coverage under Plan S begins January 1 of each year and continues until an individual’s yearly coverage allowance (84 continuous days of coverage) has been used or December 31 of the same year, whichever comes first.
Coverage from one calendar year does not extend into the next calendar year. Instead new eligibility is automatically recorded in PharmaNet for each new benefit year.
Plan S coverage cannot be provided retroactively.
What is covered?
[Updated March 17, 2017] Plan S provides 100% coverage of the cost of eligible nicotine replacement therapies up to the cost limits PharmaCare has established for these products and 100% of dispensing fee costs (up to the dispensing fee recognized by PharmaCare).
>> See the list of eligible nicotine replacement therapy products.
Procedures
For pharmacists
See Section 5.20—Smoking Cessation Program Policy.
Section 7.10 Tools and Resources
See PharmaCare’s Smoking Cessation Program web page.
Section 7.11 – B.C. Centre for Excellence in HIV/AIDS (Plan X)
General Policy Description
Plan X covers antiretroviral drugs for HIV-positive individuals through the BC Centre for Excellence in HIV/AIDS, Drug Treatment Program.
Antiretroviral medication coverage, though funded by PharmaCare Plan X, is available only through the Drug Treatment Program.
The Drug Treatment Program is designed to ensure that all medically eligible persons in B.C. who are living with HIV have access to free antiretroviral therapy.
Policy Details
Eligibility
The BC Centre for Excellence in HIV/AIDS determines an individual’s eligibility for the Drug Treatment Program. PharmaCare cannot determine eligibility for the program or enrol individuals in it.
For eligibility and enrolment information for the Drug Treatment Program, visit the BC Centre for Excellence in HIV/AIDS website.
Coverage start date
Coverage begins when an individual is enrolled in the BC Centre for Excellence in HIV/AIDS Drug Treatment Program.
Plan X coverage cannot be provided retroactively.
What is covered?
HIV-positive B.C. residents receive their antiretroviral drugs free when enrolled with the BC Centre for Excellence in HIV/AIDS, Drug Treatment Program.
Procedures
For eligibility criteria and enrolment information for the Drug Treatment Program, visit the BC Centre for Excellence in HIV/AIDS website.
As of April 2020, HIV/AIDS medications dispensed by the BC Centre for Excellence in HIV/AIDS under the Drug Treatment Program are entered in PharmaNet.
Section 7.11 Tools and Resources
The BC Centre for Excellence in HIV/AIDS website offers information about antiretroviral medication coverage under the Drug Treatment Program as well as prescribing and dispensing requirements.
Section 7.12 – First Nations Health Benefits (Plan W)
General Policy Description
Plan W covers eligible prescription drugs, certain over-the-counter (OTC) medications, and some medical supplies/devices for First Nations Health Authority (FNHA) clients.
Policy Details
Eligibility
Coverage is provided for individuals.
An individual must:
- Have active BC Medical Services Plan (MSP) coverage, and
- Be registered under the Indian Act, or be a child under 24 months of age, who has at least one parent who is registered under the Indian Act, and
- Not be an individual who is eligible to receive comprehensive drug coverage through
- a treaty and land claims agreement under the Constitution Act, 1982 (Canada) (unless that treaty and land claims agreement has been identified by the provincial Minister of Health as not resulting in ineligibility), or
- a written contribution arrangement between a First Nations organization and a government or province of Canada under which the government provides funding and which has been identified by the provincial Minister of Health as resulting in ineligibility for enrolment
PharmaCare cannot authorize Plan W coverage; eligibility for the plan is confirmed by the FNHA.
What is covered?
Under Plan W, PharmaCare pays 100% of:
- Eligible prescription drug costs up to the maximum cost recognized by PharmaCare
- Certain over-the-counter medications and devices
- Dispensing and pharmacy services fee costs up to the maximum fee recognized by PharmaCare
Note: There is no dispensing fee for non-drug over-the-counter items - Plan W benefits purchased within Canada but outside B.C. if a request for reimbursement is submitted with appropriate documentation
Coverage start date
FNHA confirms eligibility, and Plan W coverage is automatically uploaded to PharmaNet. Coverage is in effect from the time the individual’s eligibility for Plan W is uploaded to PharmaNet.
Plan W coverage cannot be provided retroactively.
Over-the-counter (OTC) medications
Plan W OTC drug benefits are eligible for 100% coverage when the claims are entered in PharmaNet, subsequent to:
- A prescriber (e.g., physician, nurse practitioner) writing a prescription, or
- A pharmacist recommending an OTC treatment:
- As part of a Minor Ailments and Contraception Service (MACS). The pharmacist must document the recommended OTC treatment on the MACS Form (PDF, 705KB). In this case, the Plan W OTC recommendation form is not required
- Outside a MACS, the pharmacist must complete the Plan W OTC Recommendation form (PDF, 548KB) or their own form that captures the same information
Pharmacists can dispense a Plan W OTC item recommended by another pharmacist. They enter the recommending pharmacist's College Registration Identification (Reg ID) in the Prescriber ID field.
For every OTC item dispensed under Plan W, the dispensing pharmacy can claim the maximum dispensing fee and mark-up covered by PharmaCare.
The dispensing pharmacy must retain a record of the OTC Recommendation form, the MACS Form or prescription, and consult with the client following the requirements outlined under the Health Professions Act, Bylaws Schedule F, Part 1 section 11(2), (3) and (4), and section 12, and the Pharmacy Operations and Drug Scheduling Act (PODSA) Bylaw section 23(1)(a), (2), (3).
Supporting FNHA clients with accessing OTC drug benefits is a component of culturally safe pharmacy care. Pharmacists are expected to explore Plan W OTC medications and provide a covered product when in the best interest of the client. Should a pharmacist decide to not recommend and/or provide an OTC drug benefit requested by an FNHA client, they should discuss therapeutic options and advise the client to seek further care as appropriate.
Frequency of dispensing
PharmaCare will not reimburse pharmacies for dispensing fees when a Plan W OTC is prescribed for daily and/or frequent dispensing.
Audit
Plan W OTC claims are subject to audit by the Ministry of Health and the First Nations Health Authority, and any amount associated with a disallowed claim will be recovered.
OTC medication resources
- First Nations Health Benefits (Plan W) OTC Drugs
- HLTH 4571 - Plan W OTC Recommendation form (PDF, 548KB)
- HLTH 5840 - MACS Form (PDF, 705KB)
Insulin products & non-drug OTC items
Insulin products
Insulin products are excluded from this policy. Insulins can be dispensed and entered in PharmaNet without a prescription or a Plan W OTC Recommendation form. Refer to Section 5.14—Insulin.
Non-drug OTC items
PharmaCare covers specific non-drug items under Plan W at their retail price up to a maximum set by FNHA, with no dispensing fee. Refer to Section 5.9—Retail Pricing Policy.
Claims for these items automatically adjudicate under Plan W and can be dispensed to a client with or without a prescription from a prescriber.
Medical supplies and equipment
Additional medical supplies and equipment (MS&E) for FNHA clients are covered by FNHA through Pacific Blue Cross (PBC).
- Refer to the FNHA Medical Supplies & Equipment Benefit page for more information, and to find a list of eligible items
Out-of-province benefits
Out-of-province purchases of Plan W benefits may be reimbursed if the client submits an HLTH 5480 - First Nations Health Benefits Out-of-Province Claim (PDF, 6KB) to PharmaCare with the appropriate documentation.
Procedures for Pharmacists
Plan W coverage not in place
Occasionally, the FNHA client eligibility may not have been uploaded to PharmaNet, and the PharmaNet transaction will not provide the expected adjudication results.
- In this case, call the PharmaCare Help Desk. The Help Desk will attempt to confirm active Plan W coverage and update PharmaNet.
- Once the Help Desk confirms that short-term assistance is in place, process the prescription as usual.
If the client has a prescription for an OTC drug
- Enter the claim in PharmaNet as you would any other prescription.
- Retain a copy of the prescription on file.
If pharmacist recommends treatment with an OTC eligible for Plan W coverage
- Complete the HLTH 4571 - Plan W OTC Recommendation form (PDF, 932KB) or another form that captures the same information.
- Enter the claim with P1 (for College of Pharmacists of BC) in the PRACT ID REF field and the College Registration Identification (Reg ID) of the recommending pharmacist in the Prescriber ID field.
- Retain a record of the purchase similar to that required for prescription items.
If an eligible OTC is recommended as a result of the Minor Ailments and Contraception Service (MACS)
- Document the recommended OTC on the MACS Form (PDF, 705KB) or another form that captures the same information.
- Enter the claim with P1 (for College of Pharmacists of BC) in the PRACT ID REF field and the College Registration Identification (Reg ID) of the recommending pharmacist in the Prescriber ID field.
- Retain a record of the purchase similar to that required for prescription items.
Section 7.12 Tools and Resources
For questions regarding eligibility for Plan W, clients and pharmacists can contact the FNHA at 1-855-550-5454 or by email at HealthBenefits@fnha.ca.
For questions regarding PharmaCare Plan W coverage and claims, pharmacies can contact the PharmaCare Help Desk, and clients should contact HIBC.
Resources:
Section 7.13 – Assurance (Plan Z)
General Policy Description
The Assurance Plan provides 100% coverage of any included product for any eligible beneficiary.
The Full Payment Policy applies to Plan Z. Providers must not charge patients any costs associated with the dispense of a Plan Z benefit.
Policy Details
Patient eligibility
All residents of B.C. with active Medical Services Plan (MSP) coverage are eligible.
Exceptional coverage
Plan Z coverage is available exceptionally in some situations.
Exceptional Plan Z coverage is processed and activated by Special Authority (SA) or Health Insurance BC (HIBC), depending on the drug. Exceptional Plan Z coverage is entered in PharmaNet (usually for 3 months; 6 months for OAT) and a response faxed back to the pharmacy or prescriber. If the call is on a weekend, HIBC enters the coverage, but still alerts Special Authority. Visit the Plan Z web page for the complete procedure.
Exceptional coverage is available for:
- Contraceptives
- B.C. residents who have completed the Application for Health and Drug Coverage (AHDC), presented their identification at an ICBC driver licensing office and are in the wait period for MSP
- up to 3 months
- Mifegymiso (mifepristone and misoprostol) for:
- B.C. residents who have completed the Application for Health and Drug Coverage (AHDC), presented their identification at an ICBC driver licensing office and are in the wait period for MSP
- up to 3 months
- Medical assistance in dying (MAiD) for:
- individuals who have public health coverage in another Canadian province or territory, or
- B.C. residents who have completed the Application for Health and Drug Coverage (AHDC) and presented their identification at an ICBC driver licensing office prior to needing MAiD but at still in the MSP wait period
- up to 3 months
- Opioid agonist treatment (OAT) for:
- B.C. residents who are not yet enrolled in MSP, or
- B.C. residents who have completed the Application for Health and Drug Coverage (AHDC) and presented their identification at an ICBC driver licensing office but are still in the wait period for MSP
- up to 6 months
- Tecovirimat for:
- patients who live in another Canadian province or territory
- B.C. residents who have completed the Application for Health and Drug Coverage (AHDC) and presented their identification at an ICBC driver licensing office but are still in the wait period
- up to 3 months
- Note: Tecovirimat is dispensed through the Product Distribution Centre only. A pharmacist or prescriber contacts HIBC at 604-682-7120 or 1-800-554-0225
Resource: Guidance for the Treatment of Monkeypox
Section 7.13 Tools and Resources
- Full procedures for exceptional Plan Z are on the Plan Z web page for health professionals
- Exceptional Plan Z Coverage eForm
- HLTH 5829 - Application for Exceptional Plan Z coverage for OAT
- HLTH 5499 - Application for Exceptional Plan Z Coverage for Mifegymiso (PDF)
- HLTH 5830 - Application for Exceptional Plan Z Coverage for Contraceptives (PDF)

8 - Pharmacy Fees, Subsidies, Provider Payment
Section 8.1 – About Pharmacy Fees and Subsidies
PharmaCare covers several fees and subsidies, within limits, that are claimed by pharmacies.
Read about the various fees and subsidies and how to claim them.
- Dispensing fees (Section 8.2)
- Frequency of dispensing fees (Section 8.3)
- Clinical services fees (Section 8.4)
- Special services fees (Section 8.5)
- Trial prescription fees (Section 8.6)
- Capitation fees (Section 8.7)
- Methadone interaction fees (Section 8.8)
- Medication review services fees (Section 8.9)
- Pharmacist administration of drugs and vaccines (Section 8.10)
- Subsidies for rural pharmacies (Section 8.11)
- Payments to providers (Section 8.12)
- Patient support fees (Section 8.13)
- Minor Ailments and Contraception Service (Section 8.14)
- Distribution of rapid antigen test kits (Section 8.15)
Section 8.2 – Dispensing Fees
General Policy Description
PharmaCare sets a maximum dispensing fee for PharmaCare beneficiaries.
All pharmacies and dispensing physicians enrolled as PharmaCare providers can claim dispensing fees.
Pharmacies can charge more than PharmaCare’s maximum dispensing fee to Fair PharmaCare beneficiaries that have not reached their family maximum.
PharmaCare limits the number of dispensing fees that can be claimed for frequent dispensing. See Section 8.3—Frequency of Dispensing Policy-Fee Limits for details.
Policy Details
Beneficiary eligibility
Beneficiaries covered under Fair PharmaCare or PharmaCare plans C, D, F, G, S, P, W and Z are eligible for coverage of dispensing fees.
The Full Payment Policy applies to all dispenses under fully paid plans (C, D, F, G, S, P, W and Z). This means the beneficiary cannot be charged a dispensing fee.
Dispensing fees, up to PharmaCare’s maximum, are applied to a beneficiary’s Fair PharmaCare deductible and family maximum. Once the beneficiary reaches their family maximum, the Full Payment Policy applies to all dispenses. This means the beneficiary cannot be charged a dispensing fee.
Dispensing fees are not covered under Plan B. PharmaCare’s monthly capitation fee for Plan B facilities covers dispensing activities for eligible benefits.
Pharmacy eligibility
Non-pharmaceutical suppliers are not entitled to charge (or claim) a dispensing fee.
The maximum dispensing fee reimbursed by PharmaCare is $11.00. Pharmacies may not claim more than $11 for a dispensing fee.
Pharmacies may not claim more than the actual fee charged to any person or agency. For example, if they charge a beneficiary $8.00 for dispensing, they must claim no more than $8.00.
Section 8.3 – Frequency of Dispensing Policy - Fee Limits
General Policy Description
PharmaCare limits the number of dispensing fees it will cover for frequent dispensing of a prescription. Frequent dispensing is defined as dispensing daily or every 2 to 27 days.
Policy Details
Fee limits
Under the Frequency of Dispensing Policy, PharmaCare limits the number of dispensing fees it covers for patients receiving:
- Daily dispensing, and/or
- Dispensing every 2 to 27 days
PharmaCare continues to cover one dispensing fee when a single fill is provided for:
- The total quantity the prescriber specified on the prescription, or
- No less than the Maximum Days' Supply allowed under PharmaCare policy
However, PharmaCare limits the number of dispensing fees covered when:
- A prescriber orders daily dispensing, or
- A prescriber orders or a pharmacist initiates dispensing in 2 to 27 day supplies
- A pharmacist renews a prescription with daily dispensing
Adherence to policy
The Ministry of Health may audit pharmacy claim records and will recover funds if the number of dispensing fees paid exceeds those allowed under this policy.
Policy inclusions/exemptions
| Opioid agonist treatments | The Frequency of Dispensing Policy applies to prescriptions for opioid agonist treatments. For methadone for maintenance, PharmaCare continues to pay an interaction fee for witnessing ingestion. |
| Plans C (Income Assistance), Fair PharmaCare, D (Cystic Fibrosis), F (Children in the At Home Program), G (Psychiatric Medications), P (Palliative Care) and W (First Nations Health Benefits) | All the plans listed are subject to the Frequency of Dispensing Policy. |
| Plan B (Long-term Care) | The Frequency of Dispensing Policy does not apply to long-term care patients covered under PharmaCare Plan B. All other plans are subject to the maximum number of fees specified below. |
| Treatment of an acute condition | PharmaCare will accept a dispensing fee if a pharmacy dispenses, in a single fill, the entire prescribed supply of a medication for a defined duration of therapy with a days' supply of 27 days or less—that is, a medication intended to treat a temporary or intermittent condition (e.g., antibiotics, antifungals, antivirals, short-term pain medications, etc.). Please refer to the Intervention Code information for details. |
| First fills of a new chemical entity as an interim supply | A pharmacy dispenses a first fill of a new chemical entity as an interim supply to last until the patient's next scheduled compliance package is prepared. Please refer to the Intervention Code information for details. |
Exclusion of inhalers, nebulizers and nitroglycerin sprays
As of October 15, 2009, certain inhalers, nebulizers and nitroglycerin sprays were excluded from the Frequency of Dispensing policy. As new products of this type become available, the list may be expanded.
See the list of excluded inhalers, nebulizers and nitroglycerine sprays (PDF, 286KB).
Inquiries about the potential inclusion of other products such as this (i.e., those that may create a quantity issue when the Frequency of Dispensing policy is applied) can be emailed to the Ministry of Health at pharma@gov.bc.ca.
Application of fee limits
Fee limits are per pharmacy. That is, if a patient fills prescriptions at more than one pharmacy on the same day, each pharmacy can claim the maximum number of fees allowed under the policy.
However, dividing patient claims between two or more pharmacies in order to circumvent the maximum number of fees covered under the Frequency of Dispensing Policy is not permitted and is subject to audit by the Ministry of Health.
The importance of entering the correct days' supply
As is the case for all claims, entering the appropriate days' supply is important.
For instance, for PRN (take as needed) medications, calculate the days' supply based on the maximum dosage per day indicated on the prescription. This prevents a patient who needs the full dosage each day from running short of medication before the expiry of the days' supply. This makes sure that the Refilling Prescriptions Too Soon Policy, Travel Supply Policy and Frequency of Dispensing Policy will be applied appropriately.
Daily dispensing
If the prescriber handwrites "dispense daily/daily dispensing"* on the original prescription, or includes the order on a prescription generated from the physician's EMR system, PharmaCare covers:
- One (1) dispensing fee per patient, per drug (DIN), per day—to a maximum of two (2) dispensing fees per patient, per day
PharmaCare covers a dispensing fee only if the date of the original prescription is no more than 100 days earlier than the dispensing date.
If a prescription is dated more than 100 days earlier than the dispensing date, the prescriber must re‑authorize daily dispensing in handwriting on a new prescription.
After a patient has reached the maximum number of daily dispensing fees, pharmacies cannot change a physician’s order for daily dispensing to every-second-day dispensing, or any other dispensing frequency, for the purposes of obtaining extra dispensing fees.
If a health authority pharmacist is given a verbal order from a physician to handwrite "dispense daily/daily dispensing"* on a prescription, PharmaCare will cover the dispensing fees when that prescription is sent to a community pharmacy.
*PharmaCare Audit cannot accept the abbreviation “DD” on the prescription.
Dispensing every 2 to 27 days
PRESCRIBER-INITIATED
If the prescriber orders medication to be dispensed in a 2 to 27 day supply in writing on the original prescription or includes the order on a prescription generated from the physician’s Electronic Medical Record system (i.e., by writing “Blister packs/packing,” “Weekly dispensing,” “Compliance packaging,” or “Bi-Weekly dispensing”), PharmaCare will cover:
- One (1) dispensing fee per patient, per drug (DIN), per prescribed supply—to a maximum of five (5) fees per patient, per prescribed supply
Example 1: If the prescriber orders weekly compliance packaging, PharmaCare covers one (1) dispensing fee per patient, per drug (DIN), per week—to a maximum of five (5) fees per patient per week.
Example 2: If the prescriber orders bi-weekly dispensing, PharmaCare covers one (1) dispensing fee per patient, per drug (DIN), every two weeks—to a maximum of five (5) fees per patient every two weeks.
Required documentation for verbal prescriptions
If a pharmacy receives verbal authorization to dispense a prescription frequently, the pharmacy will not have any documentation to support a claim for fees for frequent dispensing. To claim a fee, the pharmacy must complete a HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB), check the “2 to 27 day dispensing verbally authorized by prescriber” box, then fax the form to the prescriber.
Faxed refill authorizations
If a pharmacy transmits a refill authorization to a practitioner, the pharmacy will not have any independent documentation to show that frequency of dispensing was initiated by the practitioner. To support a claim for fees for frequent dispensing, the pharmacy must either:
- Ask the practitioner to provide the pharmacy with a refill authorization produced by the practitioner’s office requesting frequent dispensing; or
- Complete a HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB), check the “2 to 27 day dispensing verbally authorized by prescriber” box, then fax the form to the prescriber
PHARMACIST-INITIATED
If the patient meets the criteria below and the appropriate form is completed, PharmaCare will cover:
- One (1) dispensing fee per patient, per drug (DIN), per authorized supply, to a maximum of five (5) fees per patient per authorized supply
Clinical criteria guideline for a 2 to 27 day supply
For coverage of dispensing fees, a patient must be unable to manage their drug therapy independently. That is, a patient must exhibit one or more of the following:
- Cognitive impairment
- History of abuse or poor compliance
- No support structure (to assist with administration of drug therapy)
- Risk of dependence
- Susceptible to theft or loss of belongings
- Complex medication regimen
- Physical or mental disability
- Literacy issues
- Language issues
- Non-compliance or misuse is suspected
If a patient exhibits one of more of the clinical situations above, the pharmacist must:
- Complete the HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB) indicating the clinical criteria that support more frequent dispensing.
- Obtain the signature of the patient or their representative.
- Fax a Frequent Dispensing Authorization to the physician(s) who prescribed the drug(s).
- Retain proof of fax (i.e., fax verification form) on file and retain the Frequent Dispensing Authorization in accordance with College of Pharmacists of B.C. policies and bylaws. PharmaCare cannot accept forms that have been mailed or hand-delivered to the physician’s office. PharmaCare cannot accept fax reports listing multiple faxes.
A prescriber who disagrees with the frequency of dispensing can complete the last section of the form and fax it to both the pharmacy and PharmaCare. See When a prescriber disagrees with frequent dispensing for a patient for more information.
PharmaCare cannot accept Frequent Dispensing (FD) Authorization forms completed after a pharmacy has been notified of an onsite audit.
Patients with multiple dispensing frequencies or multiple prescribers
Pharmacies must notify the practitioner who prescribed the drug being dispensed (the “prescriber”). If the patient has active prescriptions from multiple prescribers, complete the HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB) indicating each prescriber, then fax the completed form to each prescriber to notify them. If the patient has more than six prescribers, complete additional Frequent Dispensing Authorization forms as needed.
If a patient is receiving medications on more than one dispensing frequency, a separate form must also be completed for each frequency.
Prescription transfers
When a prescription is transferred from one pharmacy to another pharmacy, the receiving pharmacy is responsible for completing a HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB) and faxing it to the prescriber(s).
Required annual renewal—Frequent Dispensing Authorization forms
The Frequent Dispensing Authorization form for each patient must be renewed each year, on or before the date the patient signed the original form. Download the HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB).
The prescriber name submitted with a claim must agree with the prescriber name on the HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB).
Intervention code—treatment of an acute condition
Enter the following intervention code and a fee will be covered.
| Intervention Code | UT—Treatment of an Acute Condition |
|---|---|
| Requirements | Use this code only
|
| PharmaNet Response | No response code is returned. |
| Documentation | Use of this intervention code is subject to audit. PharmaCare requires pharmacists to document the reason for the use of an intervention code in a manner accessible for audit purposes. If the documentation of the use of the intervention code is recorded on the pharmacy’s computer system, auditors require access to the pharmacy's computer system. |
Intervention code—first fill of a new chemical entity as an interim supply OR an early fill of an existing frequently dispensed prescription
| Intervention Code | DQ—Professional Fee Appropriate |
|---|---|
| Requirements | A pharmacy dispenses a first fill of a new chemical entity as an interim supply to last until the patient's next scheduled compliance package is prepared. The pharmacist must ensure that their pharmacy has not dispensed the chemical entity to the patient in the last 14 months by checking claim records and the PharmaNet profile. A change in strength, formulation and/or brand of a chemical entity already included on the patient’s PharmaNet profile does NOT constitute a first fill of a new chemical entity. The first fill of the new chemical entity must be adjudicated at least one day before the dispensing of the next compliance package. OR A patient requires an early fill of an existing frequently dispensed prescription (for instance, due to a statutory holiday). The maximum of 5 dispensing fees per authorized supply still applies; therefore, in this scenario, the intervention code must only be used up to the first 5 claims. |
| PharmaNet Response | No response. |
| Documentation | Use of this intervention code is subject to audit. PharmaCare requires pharmacists to document the reason for the use of an intervention code in a manner accessible for audit purposes. If the documentation of the use of the intervention code is recorded on the pharmacy’s computer system, auditors require access to the pharmacy's computer system. |
Intervention code—patient pay
| Intervention Code | VG—Professional Service Fee Not to be Paid |
|---|---|
| Requirements | A patient requests frequent dispensing and the pharmacist determines the patient does not meet the clinical criteria for 2 to 27 day supplies. That is, the patient does not exhibit one or more of the following:
|
| PharmaNet Response | PharmaNet will respond with DH—Professional fee adjusted. |
| Documentation | None required. |
CALCULATION OF MAXIMUM NUMBER OF FEES ALLOWED FOR 2 TO 27 DAY DISPENSING FREQUENCY
When the total number of active dispenses reaches five, no additional fees are payable.
What is an "active dispense"?
Whenever a prescription is dispensed, PharmaNet:
- Checks for fills of any medication in the previous 27 days, then
- Checks the days' supply of these previous fills, and
- Calculates the patient's remaining days' supply
If the remaining days’ supply is more than one day, the claim is considered an "active dispense."
What is not considered an active dispense?
The following are not counted as active dispenses:
- Claims for non-benefit items
- Claims for non-drug items
- Claims for patients not covered under PharmaCare
- Claims submitted with the following intervention codes:
- UT (Treatment of an Acute Condition / Full prescribed supply dispensed)
- VG (Professional Fee Not to Be Paid)
- MR (Lost or stolen)
Examples of how active dispenses are counted
When a patient receives all their medications in a weekly compliance/blister pack, the pharmacy is normally eligible for five dispensing fees for that patient each time the blister pack is dispensed. However, in some cases, patients may require medications outside the usual schedule.
Frequency of dispensing (PDF, 288KB) shows how 'active dispenses' are counted in more unusual circumstances such as when a patient has all their medication in weekly blister packs but receives some medication outside the normal dispensing schedule or when a patient has a mix of weekly blister packs and other medications.
WHEN A PRESCRIBER DISAGREES WITH FREQUENT DISPENSING FOR A PATIENT
A prescriber has the right to disagree with frequent dispensing initiated by a pharmacist. If a prescriber considers frequent dispensing to be unwarranted, PharmaCare does not cover dispensing fees. PharmaCare may recover fees paid after the date on which the prescriber notified the pharmacy.
If the prescriber disagrees with frequent dispensing, the pharmacist can choose to:
- Consult with the prescriber to ensure the prescriber is aware of any concerns, or
- Consult the patient to determine if they have another insurer who might cover the fees for frequent dispensing
If the patient chooses to pay the fees, use the appropriate intervention code to allow the patient to pay.
If you consult the prescriber and the prescriber subsequently agrees that frequent dispensing is warranted, please complete a new HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB) and fax it to PharmaCare and the physician.
If you choose not to consult the prescriber or, after consulting the prescriber, he/she does not support the frequency of dispensing, you must discontinue frequent dispensing for that patient—unless the patient chooses to continue and pay for it themselves.
PATIENTS ON DAILY DISPENSING WHO ALSO RECEIVE MEDICATION IN 2 TO 27 DAY SUPPLIES
PharmaNet adjudication allows:
- Up to two (2) dispensing fees per patient for the drugs (DINs) dispensed daily
- Up to five (5) dispensing fees per patient for the drugs (DINs) dispensed in a 2 to 27 day supply
For example, if a patient receives five prescriptions dispensed daily and six prescriptions dispensed weekly, PharmaNet will allow two dispensing fees per day for the drugs dispensed daily and five fees per week for the drugs dispensed weekly.
Please note that combining two frequencies of dispensing when treating a patient is being monitored and pharmacies may be asked to provide the supporting documentation, specifically
- For the drugs dispensed daily: the original prescription with the physician's handwritten order or the order on a prescription generated from the physician’s Electronic Medical Record system (i.e., “Dispense Daily/Daily Dispensing”) for daily dispensing.
- For the drugs dispensed in 2 to 27 day supplies: the original prescription with the physician's handwritten order or the order on a prescription generated from the physician’s Electronic Medical Record system (i.e., “Blister Packing/Packaging”, “Weekly Dispensing, “Compliance Packaging,” “Bi-weekly Dispensing”) for frequent dispensing OR the HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB).
Charges to patients
[Corrected February 20, 2020 to clarify criteria against a patient's Fair PharmaCare maximum, not deductible.]
The table below shows when a pharmacy can charge a patient or their third-party insurer.
| Patient is not covered by PharmaCare | Pharmacies can charge additional dispensing fees to patients or their third-party insurers. |
|---|---|
| Patient is below their Fair PharmaCare maximum | Only the number of fees specified in the Frequency of Dispensing Policy accumulates towards a patient's Fair PharmaCare maximum.2 Pharmacies can charge additional dispensing fees to patients or their third-party insurers. |
| Patient is above their Fair PharmaCare maximum or is on PharmaCare plan that does not have a copayment requirement | PharmaCare covers2 only the fees specified in the Frequency of Dispensing Policy. Other insurers may or may not pay additional fees, however, pharmacies cannot collect additional fees from patients. If a pharmacy's dispensing fee is more than the PharmaCare maximum fee, the pharmacy cannot collect the difference from the patient. |
| Patient's physician has not prescribed frequent dispensing and patient does not meet the criteria. Patient has requested frequent dispensing. | Pharmacies are permitted to charge patients or their third-party insurers for additional dispensing fees. The claim should be entered using the intervention code VG—Professional Service Fee Not To Be Paid. PharmaNet will respond with DH—Professional fee adjusted. |
| Dispensing fee amounts above the maximum dispensing fee PharmaCare covers | If a fee is permitted under the policy, pharmacies can charge their usual and customary dispensing fee. PharmaCare continues to cover dispensing fees up to the existing maximum allowable dispensing fee. Amounts above these limits can be charged directly to the patient. |
2Actual reimbursement of fees is subject to the rules of a patient's PharmaCare plan, including any annual maximum requirement. See Intervention Code—Patient Pay.
Questions and Answers—Policy/Dispensing Scenarios
What if a patient's frequent dispensing was discontinued and is now being resumed?
If a patient ceased receiving frequent dispensing and now requires frequent dispensing again, pharmacists must treat it as a new request. That is, for daily dispensing, pharmacists must have a properly-annotated prescription from the physician. For 2 to 27 day dispensing, the pharmacist must have a properly annotated prescription requesting frequent dispensing from the physician or must complete a HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB) and fax it to the prescriber, retaining proof of fax on file.
How do I handle travel supplies for patients receiving frequent dispensing?
Pharmacies cannot bill PharmaCare weekly for a travel supply issued on a single date. For instance, if a patient requires three weeks of compliance-packaged medications, but the full three weeks’ supply is dispensed at the same time, the pharmacy would not be permitted to claim a fee for the second and third week.
Which physician should be notified if a patient has more than one physician?
Notify each prescriber who prescribed a drug that is being dispensed frequently. If a patient has multiple prescribers and all the patient's prescriptions will be frequently dispensed (e.g., in a weekly blister pack), complete a separate HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB) indicating each prescriber (and for each dispensing frequency, if applicable) and fax the completed form to each prescriber.
Does the LZ response code always mean a fee will be deducted from the pharmacy's payment?
No. For patients below the Fair PharmaCare deductible, PharmaNet tallies the maximum number of allowable fees only to determine how much should count towards the patient's deductible. The LZ code is returned for fees above the maximum, but, as no dispensing fee would have been covered anyway, it makes no difference to the pharmacy's payment.
Is the Refilling Too Soon policy still in place?
Yes. The Refilling Too Soon policy introduced in 2002 is still in place. Under this policy neither the drug cost nor the dispensing fee is covered if a patient has more than 14 days' supply of medication left from the previous fill. This policy applies to all medications whether or not they are considered "frequently dispensed."
The Travel Supply Policy introduced in 2008 also still applies. PharmaCare continues to allow an earlier fill once every six months (180 days) if a patient will be travelling outside B.C. Patients can top up their supply to the maximum days’ supply recognized by PharmaCare as long as they complete and sign a Travel Declaration form (supplied by the pharmacy) on the date their prescription(s) is filled.
Does PharmaCare accept a prescriber's computer-generated prescription (i.e., not handwritten) that authorizes frequent dispensing?
Yes. A physician’s order for frequent dispensing included in a prescription generated from the physician’s Electronic Medical Record system is acceptable.
Section 8.3 Tools and Resources
| Dispensing frequency scenario | Limit on # of dispensing fees? | Authorization form required? | Notes |
|---|---|---|---|
| Daily | Yes. Maximum of two dispensing fees per patient per day. | No | Applies to all plans except for Plan B. Prescriber must handwrite “Daily Dispensing” on the prescription or include the order on a prescription generated from their Electronic Medical Record system. |
| 2 to 27 day supply, prescriber has ordered dispensing frequency | Yes. Maximum of five fees per patient, per prescribed supply (i.e., the prescribed frequency—weekly, bi‑weekly, etc.). | No | Applies to all plans except for Plan B. Prescriber must handwrite “Blister packs/packing,” “Weekly Dispensing,” or “Compliance Packaging” on the prescription or include the order on a prescription generated from their Electronic Medical Record system. |
| 2 to 27 day supply, pharmacist has initiated dispensing frequency | Yes. Maximum of five fees per patient, per prescribed supply (i.e., the prescribed frequency—weekly, bi‑weekly, etc.). | Yes. Pharmacist must fax a completed HLTH 5378 - Frequent Dispensing Authorization (PDF, 543KB) form for each dispensing frequency to each prescriber and retain proof of fax on file. | Applies to all plans except for Plan B. |
| 28 day supply or more | No | No | Dispensing should be in keeping with the PharmaCare Maximum Days’ Supply policy (30 days for short-term medications and for the first fill of a long-term medication; 100 days for subsequent fills of a long-term medication). The PharmaCare Refilling Too Soon and Travel Supply policies continue to apply. |
Section 8.4 – Clinical Services Fees
General Policy Description
PharmaCare pays pharmacies enrolled as PharmaCare providers set amounts for providing clinical services associated with prescription adaptation by a pharmacist for residents of B.C.
Clinical services fees can be claimed whether or not the drug or the patient is covered by PharmaCare.
The maximum amount PharmaCare reimburses a pharmacy for any combination of medication reviews, clinical services (e.g., prescription adaptation), and drug and vaccine administrations for the same patient, on the same day is $78.00.
Policy Details
Eligibility
Any pharmacy that is a PharmaCare provider can claim the clinical services fee. The client they provide the clinical service to does not need to be covered by PharmaCare.
Prescription adaptation
Fees can only be claimed for prescription adaptation as defined by the College of Pharmacists of BC Professional Practice Policy 58 (PDF, 148KB):
- Renewing a prescription
- Changing the dose, formulation or regimen of a prescription
- Making a therapeutic drug substitution within the same therapeutic class
PharmaCare may recover clinical services fees paid for services that are not consistent with the PPP-58 definition of prescription adaptation.
Clinical services fees must be claimed when a clinical service has been performed. For adaptations, a clinical services fee can be claimed on the first dispense of the adapted prescription, and not on any refills.
PPP-58 updates
In October 2022, the period of validity for a prescription was extended to 2 years from the written date, and pharmacists were authorized to adapt prescriptions for a wider range of drugs and conditions.
In August 2024, the college updated PPP-58 to authorize pharmacists to:
- Adapt prescriptions that have previously been adapted
- Adapt a prescription from a former practitioner (i.e. a practitioner who is no longer practising), if the prescription at the time of adaptation is otherwise valid
- Renew prescriptions for a narcotic, controlled drug, or targeted substance for a duration that may be longer than prescribed, for continuity of care
- Change the dose, formulation or regimen for a prescription for a narcotic, controlled drug, or targeted substance, if the quantity dispensed does not exceed the stated amount authorized in the prescription
For a pharmacist to renew a prescription, the following must apply:
- The prescription was written less than 2 years ago
- The condition being treated is stable
- There has been no clinically significant changes to the prescription for a minimum of 3-6 months per accepted clinical practice for the condition being treated
The College of Pharmacists of BC’s Professional Practice Policy 58 (PDF, 148KB) details the principles and protocols for all prescription adaptations. The college’s website provides detailed information about, for example, what qualifies as an adaptation, obtaining patient consent, the difference between adapting and providing an emergency supply, how to notify prescribers and how to document adaptations.
Ineligible claims
A clinical services fee cannot be claimed:
- For emergency fills
- For emergency contraceptives
- For refills of an adapted prescription. Only a dispense fee can be claimed for refills
- For contacting a physician to clarify a concern, amend a prescription, or dispense an interchangeable drug
Maximum clinical services fee
The maximum clinical services fee for adaptation is
- $10.00 for a prescription renewal or for changing a dose, formulation, or regimen
- $17.20 for making a therapeutic substitution
Maximum number of fees
The Ministry pays a maximum of two clinical services fees per drug, per person during a six-month period.
Payment schedule
Clinical services fees are paid monthly.
Full Payment Policy
The Full Payment Policy applies to clinical services fees. This means that pharmacies cannot solicit or accept additional payment from a patient or any other party for services associated with prescription adaptation. If the pharmacy requests or accepts any such fees or payments, PharmaCare may recover all clinical services fees paid to the pharmacy and may refuse to pay additional clinical services fees to the pharmacy.
Relationship to dispensing fees and special services fees
Special services fees cannot be claimed for a prescription for which a clinical services fee is being claimed.
If the situation is consistent with the College of Pharmacists of BC’s Professional Practice Policy 58, and the pharmacist is adapting the prescription in a situation in which the alternative is to refer a patient back to the prescriber, it is appropriate to claim a clinical services fee. If however, the pharmacist decides it is in the patient’s best interest to refer them to the prescriber without filling the prescription, the pharmacist can not claim a clinical services fee, but may be eligible for a special services fee, if the prescription would have been a PharmaCare benefit for that patient.
Procedures
Submitting claims for prescription adaptation
Clinical services fees are paid based on the number of claims a pharmacy submits with specific intervention codes (below). Do not submit the clinical services fee amount with the claim.
To submit a claim for a clinical services fee:
- PRACT ID Ref field: enter P1 (for College of Pharmacists of BC)
- PRACT ID field: enter your College ID.
- SIG (directions) field: enter Adapted at the beginning of the field (unless leveraging PharmaNet PPM version 70 software, which automatically marks the record as an adaptation), and additional directions after.
- Include the appropriate clinical services fee intervention code. Clinical services fees will not be paid for claims that do not have the appropriate intervention code.
| Code | Description |
|---|---|
| NI | dosage change |
| NJ | formulation change |
| NK | directions for use modified |
| NL | renewal of prescription |
| NM | therapeutic substitution |
Reconciling clinical services claims with payments
Pharmacies can call the PharmaCare Help Desk to find out whether a claim for clinical services has been accepted (paid) or rejected (not paid).
Help Desk staff can access payment and claim details for specific pay periods, and can fax a copy of these details (with patient identifiers removed) if requested.
Procedure for entering emergency fill and emergency contraceptive claims
Use the following procedure to submit claims for an emergency fill or for emergency contraceptives. These claims are not eligible for a clinical services fee.
- In the PRACT ID Ref field, enter P1 — College of Pharmacists of BC.
- In the PRACT ID field, enter your College ID.
- Include the appropriate intervention code.
| Code | Description |
|---|---|
| NN | emergency supply of medication |
| NO | emergency contraceptive |
Section 8.4 Tools and Resources
Section 8.5 – Special Services Fees
General Policy Description
Sometimes a pharmacist may choose not to dispense a prescription for reasons such as a drug-to-drug interaction or suspicion of multi-doctoring.
In some “refusal to fill” situations, the pharmacist may be entitled to claim a PharmaCare fee for “special services.”
Policy Details
When a special services fee can be claimed
The original prescription must be entered and reversed before a special services fee can be claimed. (See Procedure for details.)
PharmaCare pays a special services fee to any PharmaCare provider who does not dispense a prescription after reviewing information on PharmaNet, when the PharmaCare paid amount for the original prescription is greater than $0.00. Special-services fee claims must be submitted on the same day as the reversal.
When a pharmacist refuses to fill a prescription for suspected poly-pharmacy/ multi-doctoring and processes a claim for a special services fee, the prescription can be returned to the patient. To prevent the patient from presenting the prescription to another pharmacist, mark the prescription with the notation “refused to fill” and the date before returning it.
Following are situations in which a special-services fee may be claimed. The pharmacist must provide appropriate justification for the refusal-to-fill by using one of the applicable Canadian Pharmacists Association (CPhA) intervention codes. The approved CPhA intervention codes are:
| Description | CPhA Code |
|---|---|
| Significant drug interaction (drug-to-drug) | CI |
| Prior adverse reaction | CA |
| Therapeutic duplication | CD |
| Sub-therapeutic dose | CL |
| Dangerously high dose | CH |
| Treatment failure | CB |
| Potential overuse/abuse | CO |
| Suspected polypharmacy/multi-doctoring | CM |
| Falsified/altered prescription | CF |
| Consulted prescriber—changed dose | UB |
| Consulted prescriber—changed instructions for use | UC |
When a special services fee cannot be claimed
A refusal-to-fill in response to a patient asking for an early fill of a prescription does not qualify for a special services fee.
Fees may not be claimed for repeat occurrences involving the same individual at the same pharmacy within a short time.
Because PharmaCare makes capitation payments for those living permanently in long-term care (PharmaCare Plan B patients), it does not pay special services fees for Plan B claims.
Special services fee—maximum allowable fee
The maximum special services fee paid is twice the amount of the pharmacy’s normal PharmaCare dispensing fee, at the time of the dispensing request.
Audit
Please note that Special Services fees are subject to audit by PharmaCare.
Procedures for pharmacies
Pharmacies using version 3 software
- Submit the original prescription.
- Reverse the prescription using one of the intervention codes above on the same day as the prescription fill.
- Submit the claim again on the same day as the reversal and with the following information:
- The special services fee code (consult your vendor for information if necessary)
- Intervention/exception codes
- $ amount of the special services fee (in the ZCD segment)
Pharmacies using version 70 software (updated to support Provincial Prescription Management [PPM])
- If the prescription authorization has not been uploaded to PharmaNet (i.e., for paper or faxed prescriptions received at the pharmacy), upload the prescription with the details as written by the prescriber. Do not perform a dispense/claim adjudication.
- Update the prescription authorization status to 'O – Obsolete' by submitting a TRX-X2 transaction.
- Ensure the following are entered as this information is used by PharmaCare to provide payment to pharmacies:
- Reason code = RF (Refusal to Fill)*
- Medication Management Intervention (MMI) code = RTF (Refusal to Fill)*
- Clinical Service (CS) code - as appropriate, refer to table below
| CS code | Description |
|---|---|
| ATPR | ALLERGY TO PRODUCT IS ON RECORD |
| CGDS | CONSULTED PRESCRIBER – CHANGED DOSAGE |
| CGIN | CONSULTED PRESCRIBER – CHANGED INSTRUCTIONS FOR USE |
| DNGR | DANGEROUSLY HIGH DOSE |
| DUPL | THERAPEUTIC DUPLICATION |
| FALS | FALSIFIED/ALTERED PRESCRIPTION |
| MULT | MULTI-DOCTORING/MULTI-PHARMACY |
| PADR | PRIOR ADVERSE REACTION |
| PINE | PRODUCT IS NOT EFFECTIVE |
| PTOA | POTENTIAL OVERUSE/ABUSE |
| QPNR | QUANTITY PRESCRIBED IS NOT RATIONAL |
| SDTD | SIGNIFICANT DRUG INTERACTION (DRUG-TO-DRUG) |
| SUBD | SUB-THERAPEUTIC DOSE |
| TFLR | TREATMENT FAILURE |
*Note: Some software may automatically populate the Reason and MMI codes based on workflow prompts and selections by user.
Performing this action will prevent the prescription authorization from being filled in the future. It is important the pharmacist only takes this action for prescriptions that are true refusals to fill and not a situation where a client is requesting an early fill of a medication. For additional questions about entry procedures, pharmacies are encouraged to contact their software vendor.
Section 8.6 – Trial Prescription Program
General Policy Description
The intent of the Trial Prescription Program is to reduce the drug wastage that results when individuals have adverse drug reactions and cannot use the remainder of a normal prescription.
Under the program, the pharmacist can initially dispense a smaller quantity (maximum 14-day supply) than the prescription indicates for specific high-cost medications with known high incidence of side effects.
Policy Details
Pharmacy participation in the Trial Prescription Program is voluntary, but encouraged.
A small trial quantity (maximum 14-day supply) may be dispensed to individuals who are—for the first time—receiving one of a specific selection of chronic-condition medications that has a documented high incidence of side effects.
>> See the PharmaCare website for a list of the drugs eligible under the Trial Prescription Program.
The balance of the prescription may be dispensed once it has been established that the patient can tolerate the medication. That is, if the 14-day trial prescription produces no side effects, the patient returns to the pharmacy to fill the balance of the prescription.
The pharmacy can claim a second dispensing fee for filling the balance of the prescription.
Coverage of the dispensing fee (up to the PharmaCare maximum dispensing fee) and eligible drug costs for both the trial quantity and subsequent fill, if any, is subject to the patient's usual PharmaCare plan rules.
Payments the patient makes towards the eligible dispensing fee and eligible drug cost of both the trial quantity and subsequent fill, if any, will count towards the patient's Fair PharmaCare deductible.
Procedures
PROCEDURES FOR PHARMACIES
Dispensing a trial quantity
- Verify the medication is eligible under the Trial Prescription Program.
- Use the intervention code MT to designate a trial prescription in PharmaNet.
- Submit the claim in PharmaNet with the actual quantity of medication dispensed.
- From the patient, collect any applicable contribution toward the dispensing fee and drug cost.
- Indicate "Trial Prescription" directly on the label/receipt.
- If unsure of a trial drug, call the PharmaCare Help Desk to determine if a particular DIN is eligible under this program.
Dispensing the balance of a prescription
- Put the balance of the prescription through as a normal transaction using:
- The same prescription number as for the trial quantity, and
- The balance of the prescription as the quantity, and
- A blank in the Intervention and Exception Code field.
The usual rules for days' supply will be applied by PharmaNet in the adjudication.
- Collect any applicable co-payment from the patient.
Completion of a form documenting the trial prescription is no longer required.
Section 8.7 – Capitation Fees for Plan B (Long-term Care)
General Policy Description
PharmaCare Plan B covers B.C. residents who are permanent residents of long-term care facilities licensed under the Community Care and Assisted Living Act and patients of hospitals licensed under Part 2 of the Hospital Act.
Policy Details
Eligible facilities
PharmaCare Plan B covers British Columbians who are permanent residents of long-term care facilities (excluding extended-care, acute-care, multi-level and assisted living facilities) that are licensed under the Community Care and Assisted Living Act and patients of hospitals licensed under Part 2 of the Hospital Act.
Payment process
Each long-term care facility is served by one contracted pharmacy.
To receive payment for services to a long-term care facility, a pharmacy must be enrolled with PharmaCare in the Plan B pharmacy sub-class. See the PharmaCare Provider Enrolment Guide for more information.
Pharmacies servicing long-term care facilities provide residents with medications packaged in a monitored dosage system.
PharmaCare pays a monthly fee (capitation fee) to pharmacies contracted to provide service to a long-term care facility for each bed occupied by a patient receiving Plan B coverage. See Related Services List for what is included in the capitation fee.
Capitation fee
At the end of each month, PharmaCare pays the contracted pharmacy a capitation fee for each bed that
- The pharmacy has serviced, regardless of the level of service provided, and
- Was occupied by a recipient of PharmaCare Plan B coverage
The capitation fee is $65. The capitation fee is paid in addition to eligible drug costs.
Payments are per serviced bed, regardless of whether different residents have occupied a bed during the month.
Capitation fee payment is based on the actual occupancy of the facility (i.e., the actual number of occupied beds for the month), not on the maximum licensed capacity of the facility.
Capitation fees are not made for short-term (“respite”, “swing” or “temporary”) patients in a facility. Claims for pharmacy services for respite patients must be made under Fair PharmaCare or Plan C, depending on each patient’s eligibility.
Plan B payments are monitored. Any over-payment in a given month will be recovered. All PharmaCare payments are subject to audit by the Ministry of Health.
All supporting documentation for Plan B invoices (e.g., working papers, prescriptions, authorizations, and the PHN list for which claims were made on the invoice) must be retained on file by the pharmacy in accordance with the Audit Policy.
Relationship to other fees
No dispensing fees or special services fees are paid for Plan B patients. These are covered by the capitation fee.
Pharmacists may charge a fee for administering a publicly funded vaccine to a Plan B patient. For details, see Section 8.10—Pharmacist Administration of Drugs and Vaccines.
Changes in facility licence, licensee or administration
The British Columbia PharmaCare Pharmacy Agreement for the Provision of PharmaCare Services to Long Term Care Facilities signed for long-term care is valid only for the licensed facility named in the agreement.
In the event of a change in the facility licence or licensee (e.g., a change in facility name, address, etc.), the pharmacy must
- Obtain a new “Appointment of Pharmacy Services” agreement from the facility; and
- Sign a new British Columbia PharmaCare Pharmacy Agreement for the Provision of PharmaCare Services to Long Term Care Facilities
If there is a change of administration at a long-term care facility, the facility must
- Provide the pharmacy with a new “Appointment of Pharmacy Services” agreement
Procedures for Pharmacies
Invoicing

Submit only one invoice per pharmacy per month for Plan B capitation fees (i.e., all contracted care facilities must be claimed on the same invoice). Multiple invoices cannot be processed.
Invoices for Plan B must be submitted on a monthly basis, using PharmaCare Prescription Invoices.
To order additional Invoices, contact the PharmaCare Help Desk.
Submit Plan B monthly invoices to PharmaCare at or near the end of each month. This ensures that the information on the number of occupied and serviced beds for the month is accurate.
To submit an invoice for Plan B
- On a PharmaCare Prescription Invoice, complete the following:
- Pharmacy identification (i.e., name and address of the pharmacy)
- Current date
- Number of claims submitted (i.e., number of claimed beds)
- Pharmacy code
- Total $ amount invoiced.
- Write "Nursing Home Beds" at the top of the invoice.
- Write the month to which the invoice applies at the top of the invoice.
This is necessary as the "Current Date" in step 1 does not always reflect the month of service.
- Sign the invoice and include your position in the pharmacy.
Unsigned invoices will be returned by PharmaCare without payment.
- Mail the invoice to
PharmaCare Help Desk
PO Box 9655 Stn Prov Govt;
Victoria BC V8W 9P2
Section 8.7 Tools and Resources
For questions about Plan B billings, contact PharmaCare Help Desk.
To order PharmaCare Prescription Invoices, contact the Help Desk and provide your Site ID.
Section 8.8 – Methadone Maintenance Payment Program
General Policy Description
Under the Methadone Maintenance Payment Program, PharmaCare offers pharmacies a payment program for witnessing ingestion of methadone.
This section also includes information on reimbursement and claims submission for methadone for treatment of pain.
Policy Details
Eligible activities for Methadone Maintenance Payment Program
PharmaCare offers pharmacies a payment program for pharmacy-witnessed ingestion of only methadone for maintenance.
Eligible products are detailed on the OAT DINs and PINs web page.
Methadone—coverage for pain
Methadone is a regular benefit under most PharmaCare plans. Methadone prescribed for pain must be dispensed using the Drug Identification Number (DIN) for the product dispensed.
Required Product Identification Numbers (PINs)
Pharmacies must use specific PINs and DINs to identify claims for methadone for maintenance and those for the treatment of pain.
[List Updated May, 2019] See the OAT DINs and PINs web page.
General requirements for the Methadone for Maintenance Payment Program
Participation in the interaction fee portion of the payment program is optional. Pharmacies that elect to participate must
- Enrol in the Opioid Agonist Treatment Provider sub-class (for more information, see the PharmaCare Provider Enrolment Guide.
- Undertake not to bill patients more than the amounts reimbursed by PharmaCare
- Agree not to offer cash or incentives of any kind to methadone clients. Without limiting the generality of the foregoing statement, incentives include, but are in no way limited to, air miles, loyalty points and bus passes.
Drug cost reimbursement and fees paid
Methadone for maintenance
Pharmacies that choose to participate in the payment program are reimbursed for pharmacist-witnessed ingestion of eligible methadone products at:
- The maximum price PharmaCare covers for the drug, plus
- The usual dispensing fee, plus
- An interaction fee of $7.70 for each dispensation involving direct interaction with the patient
Pharmacies that do not enroll in the payment program are reimbursed for eligible methadone products at:
- The maximum price PharmaCare covers for the drug, plus
- Their usual dispensing fee only
Methadone dispensing fees and the Frequency of Dispensing Policy
Methadone dispensed under the Methadone Maintenance Payment Program is subject to a maximum of one dispensing fee and one interaction fee per patient per day (in cases where the interaction fee is applicable), regardless of physician administration instructions on the prescription.
Dispensing fees for methadone for maintenance are subject to the Frequency of Dispensing policy, however, PharmaCare continues to pay an interaction fee for each ingestion witnessed.
Methadone dispensations involving direct interaction with the patient
To qualify for methadone interaction fee, the pharmacist must witness the ingestion of the medication by the patient.
In the context of OAT delivery only, the pharmacist can appoint a delegate to witness the ingestion of OAT and qualify for the interaction fee. The delegate must be a pharmacy employee and can be a pharmacy assistant, pharmacy technician, or a pharmacy nurse.
Methadone dispenses involving direct interaction with the patient must be dispensed under the correct OAT product identification numbers (PINs).
Methadone dispensations that do NOT involve direct patient interaction
The interaction fee may not be claimed when a pharmacist does not witness the ingestion.
If a pharmacy claims the interaction fee when a pharmacist has not witnessed ingestion, the claim is subject to audit and recovery.
Situations that do not involve direct interaction include:
- Dispensing to someone who is incarcerated or in a pre-trial facility
- Dispensing to someone covered by Plan B
- Dispensing to another health care provider (e.g., physician or nurse) for administration to a patient at a clinic
- Daily pick-ups by patients without a pharmacist witnessing ingestion
- Dispensing of “carries” (witnessing the ingestion and dispensing a carry must be submitted in a single claim)
Methadone dispensations for OAT that do not involve direct patient interaction must be dispensed under the appropriate OAT PINs.
PharmaCare does not pay an interaction fee for dispenses of buprenorphine/naloxone, Suboxone, or Kadian.
Multiple-day supply of methadone ("carries")
“Carries” must be claimed as a multiple-day supply for drug cost, plus a single dispensing fee and single interaction fee.
Multiple dispensing fees and/or interaction fees claimed for “carries” will be subject to recovery by PharmaCare.
For example, when a pharmacist dispenses a three‑day prescription for methadone, and there is face-to-face interaction with the patient in the form of witnessing ingestion, only one dispensing fee and one interaction fee is permitted for the three-day supply.
Payment of interaction fees
The interaction fee is payable to enrolled pharmacies for all PharmaCare-eligible claims (except for Plan B claims), including those above or below the Fair PharmaCare deductible.
Interaction fees are calculated automatically using transaction data from PharmaNet. Payments are made monthly on the last payment of the following month.
Reimbursement for specific PharmaCare plans and patient groups
Plan B:
Methadone for maintenance patients covered under Plan B must also be dispensed under the appropriate product identification numbers for methadone for maintenance and are reimbursed as follows:
- Actual acquisition cost to the maximum price PharmaCare covers for the drug.
- Usual Plan B capitation rates will be paid.
Fair PharmaCare:
PharmaCare pays methadone interaction fees for Fair PharmaCare patients regardless of whether they have met their annual deductible.
Persons born before 1940:
PharmaCare covers 100% of the cost of methadone for maintenance and associated dispensing fees for persons born before 1940, whether or not they are registered for Fair PharmaCare.
Persons born on or after January 1, 1940, do not qualify for this coverage even if their spouse receives enhanced Fair PharmaCare coverage.
This fee coverage applies only to Methadone Maintenance Payment Program dispensing fees.
Pharmacies can process methadone maintenance transactions as normal for these patients; PharmaCare automatically pays the dispensing fees for eligible seniors on those transactions.
Non-Insured Health Benefits (NIHB) clients:
PharmaCare does not cover methadone for clients of the Non-Insured Health Benefits (NIHB) program.
The NIHB program covers the cost of methadone for eligible clients. Methadone is an open benefit under the NIHB.
For more information, pharmacies can contact the NIHB Toll-free Inquiry Centre (First Canadian Health Management Corporation) at 1-888-511-4666, Monday to Friday, 6:30 am to midnight (EST) and, on weekends and holidays, from 8 am to midnight (EST).
Audit of methadone claims
The accuracy and validity of methadone claims is verified on a monthly basis.
Reminder—Batch Claims Policy:
As stated in the PharmaNet Compliance Standards, it is acceptable to submit batch claims only if you are submitting claims for
- Long-term care facility patients
- Prescriptions filled during a network outage (within 24 hours of reconnecting to the network)
Procedures
Entering claims for methadone
Enter the claim using the correct PIN (for maintenance) or DIN (for pain). Refer to the OAT DINs and PINs page for detailed instructions for specific products/strengths and interaction fees.
Section 8.9 – Medication Review Services
General Policy Description
B.C. pharmacies can submit a claim to PharmaCare for medication review services provided by pharmacists to eligible patients.
A medication review is a patient-care service that seeks to enhance a patient’s understanding of their medication regimen, identify and resolve drug therapy problems, and improve health outcomes.
The service is provided by a pharmacist through a one-on-one, in-person appointment during which the patient and pharmacist identify all medications that the patient is taking, discuss how the medications are best taken and, where appropriate, create a medication management plan to address any drug therapy problems. At the end of the appointment, the pharmacist provides the patient with one or more documents listing their medications.
The maximum amount PharmaCare reimburses a pharmacy for any combination of medication reviews, clinical services (e.g., prescription adaptation), and drug and vaccine administrations for the same patient, on the same day is $78.00.
This policy standardizes how medication review services are delivered across B.C.
There are four types of medication review services:
| 1. Medication Review—Standard (MR-S) | Provided at community pharmacies and eligible for PharmaCare payment. |
|---|---|
| 2. Medication Review—Pharmacist Consultation (MR-PC) | |
| 3. Medication Review—Follow-up (MR-F) | |
| 4. Medication Review—Primary Care Network (MR-PCN) | Provided by pharmacists in a primary care network. No PharmaCare payment is provided. |
Policy Details
POLICIES APPLICABLE TO ALL MEDICATION REVIEW SERVICES IN COMMUNITY PHARMACIES
Pharmacists should ensure they are familiar with the entire contents of this section of the PharmaCare Policy Manual before delivering and submitting claims for a medication review service.
This section contains policies applicable to medication review services in community pharmacies. (For policies and activities applicable to medication reviews provided by clinical pharmacists in primary care networks, see Medication Review—Primary Care Network [MR-PCN]):
1. Determining patient eligibility
Before performing a medication review service for a consenting patient, for which a claim will be submitted, pharmacists must ensure the patient is eligible for PharmaCare coverage of that service.
To be eligible to receive any of the three PharmaCare-paid medication review services (including follow-up appointments), the patient must meet all the criteria in the table below.
|
The patient must |
Notes |
|---|---|
|
Be a resident of B.C. |
Must have a permanent address in B.C. verified by a B.C. driver’s licence, BC Services Card or other ID card. |
|
Have a B.C. Personal Health Number (PHN) |
Any B.C resident with a PHN is eligible, even if they aren't covered by or registered with PharmaCare, such as Non-Insured Health Benefits and Canadian Armed Forces beneficiaries. |
|
Not be covered under PharmaCare Plan B |
Medication review services for individuals in long-term care facilities are already funded as part of the monthly capitation fee for PharmaCare Plan B facilities. |
|
Have at least five different qualifying medications that have been entered into PharmaNet
|
>> See Qualifying medications below for details. |
|
Have a clinical need for service |
>> See Clinical need below for details. |
|
Have not exceeded the allowable number of medication review services |
>> See Allowable number of medication review services below for details. Pharmacists are responsible for checking the patient’s PharmaNet record for the number of reviews received. |
|
Sign the acknowledgement on the Best Possible Medication History (BPMH) form |
>> See 3. Obtaining patient signature in acknowledgement section for details. |
Individual DINs and PINs may be counted only once.
A qualifying medication is one of the following that has been entered into PharmaNet:
- A prescription medication; that is, a Schedule 1 (prescription required) drug
- A compounded prescription medication (with a discrete PIN)
- Insulin (if the patient takes multiple types of insulin, it counts as only one qualifying medication)
Non-qualifying items
Items that do not qualify include:
- All non-prescription products, with the exception of insulin, whether or not they are covered by PharmaCare, including but not limited to: over-the-counter medications; vitamins and nutritional supplements; vaccines (regardless of whether they are privately or publicly funded); non-prescription compounds; and natural/homeopathic products.
- Prescriptions with a Discontinued status in PharmaNet
- Prescriptions that have been reversed in PharmaNet
- Prescriptions with a Not Filled status in PharmaNet
- Non-drug supplies including but not limited to:
- blood glucose testing supplies (e.g., continuous/flash glucose monitors, strips, lancets, needles)
- insulin pumps and insulin pump supplies (e.g., infusion kits)
- medical equipment and supplies (e.g., orthoses, prostheses, gloves)
Clinical need
When determining a patient's eligibility to receive medication review services, clinical need must be identified and clearly documented as one or more of the following:
- Prescriber has requested a medication review
- Patient has multiple diseases
- Patient has one or more chronic diseases
- Patient's medication regimen includes one or more non-prescription medications
- Patient's medication regimen includes one or more natural health products (NHPs)
- Patient has a drug therapy problem (DTP) (see below)
- Patient was recently discharged from hospital
- Patient has multiple prescribers
- Patient is receiving medication(s) that require laboratory monitoring
The seven types of drug therapy problems (DTPs) are:
- Unnecessary drug
- Needs additional drug
- Ineffective drug
- Dosage too low
- Dosage too high
- Adverse drug reaction
- Patient self-management (non-adherence)—that is, the patient is not taking the drug appropriately
Allowable number of medication review services
Eligible patients may receive coverage for
- Either one Medication Review—Standard (MR-S) or one Medication Review—Pharmacist Consultation (MR-PC) service (but not both) every 6 months*
AND
- Up to four Medication Review—Follow-Up (MR-F) services every 12 months
*A patient is not eligible for coverage of an MR-S or MR-PC if they have received an MR-PCN within the past 6 months. Patients are still eligible for up to 4 MR-Fs in the community following MR-PCNs, within 12 months.
>> For specific eligibility requirements for each medication review service, refer to the Required Activities for MR-S, Required Activities for an MR-PC, and Required Activities for an MR-F
Patients who receive medication review services from different pharmacies (including PCNs) are still subject to the coverage limits described above (i.e., coverage limits are per patient not per pharmacy).
To ensure coverage is available, pharmacists should review a patient's PharmaNet profile to determine whether the patient has reached their maximum number of allowable medication review services (MR-S, MR-PC, MR-F) before they conduct the medication review.
Medication review service claims in excess of the maximum allowable will not be reimbursed even if the claims are submitted by different pharmacies.
PharmaNet cannot reject medication review service claims in excess of the maximum allowable at the time of submission. These claims are adjudicated in monthly batches. Any claims in excess of the maximum allowable found at that time will be disallowed.
2. Documenting medication review services
PharmaCare requires pharmacies that submit a claim for medication review services to retain specific documentation to support their claim.
Documenting medication review services:
- Provides auditable proof that an eligible medication review service occurred
- Provides patients, caregivers, and other healthcare professionals with accurate, complete, and current information about a patient's medications
The two forms PharmaCare requires for use in documenting medication review services are:
|
Best Possible Medication History (BPMH) (Word, 282KB), including:
|
Required for all medication review services in community pharmacies |
|
Required whenever a pharmacist identifies and/or takes action to resolve a patient's DTP |
|
|
Optional |
The content of these forms constitutes the minimum acceptable documentation required for PharmaCare coverage of a medication review service claim. The associated claim is subject to recovery if these documentation requirements are not met.
>> For details, see the Required Documentation section for each medication review service: MR-S Required Documentation, MR-PC Required Documentation, and MR-F Required Documentation.
PharmaCare provides templates for all medication review services forms provided in community pharmacies. Pharmacies may use the templates to record required information (see Form templates under Tools and Resources below) or create their own forms.
Pharmacies that create their own forms must ensure those forms contain all the text and field titles as well as all the fields shown in the PharmaCare version. For details, see If you are creating your own forms.
Document retention and storage
Documents must be retained in the same manner as other patient records.
>> For more information, see the PharmaCare Policy Manual, Section 10–Audit.
3. Obtaining patient signature in acknowledgement section
PharmaCare covers medication review services only if the patient or their legal representative signs the acknowledgement on the Best Possible Medication History (BPMH) (Word, 282KB) form at the conclusion of the medication review service.
Whenever someone else is acting on a patient’s behalf, the pharmacy must retain documentation of that person’s right to act as the patient’s legal representative.
For each medication review service provided, the patient or their legal representative must sign acknowledgement on the BPMH form.
In B.C., an acceptable legal representative for a patient is a representative acting under a representation agreement under the Representation Agreement Act or a committee appointed under the Patients Property Act.
Note: The Health Professions Act (HPA) and Pharmacy Operations and Drug Scheduling Act (PODSA) bylaws state that, for purposes of continuity of care, pharmacists can share information about a patient with other healthcare professionals within the circle of care without having to obtain specific consent from the patient to do so.
>> See HPA Bylaws (PDF, 611KB), section 71 (Use of Personal Information) and section 72 (Disclosure of Personal Information) and PODSA Bylaws (PDF, 354KB), section 35 (Data Collection, Transmission of and Access to PharmaNet Data) and section 36 (Confidentiality).
4. Claiming medication review service fees
Pharmacies must not request or accept additional fees or payments from any patient or third-party payer in relation to a medication review service for which a fee will be, or has been, claimed from PharmaCare.
Only one fee (i.e., MR-S, MR-PC or MR-F fee) can be claimed for each service appointment.
The maximum PharmaCare reimburses for a combination of medication review services, clinical services, or administration of vaccines for the same patient, on the same day, from the same pharmacy is $78.00.
Example: If a pharmacy claims an MR-PC, that pharmacy cannot be reimbursed for any other service on that day or if a pharmacy submits a claim for an MR-S, a therapeutic substitution and administration of a vaccine on a single day, only the MR-S and vaccine administration will be eligible for reimbursement.
To ensure maximum reimbursement, and to preserve the accuracy of the patient’s medication history, please submit all claims whether or not you expect the claim to be reimbursed.
If a pharmacy provides multiple services to a patient on the same day but submits claims on separate days in an attempt to circumvent this policy, any claim reimbursed in excess of the $78.00 daily limit is subject to recovery by PharmaCare.
>> See MR-S–Claims for Payment, MR-PC–Claims for Payment, and MR-F–Claims for Payment.
REQUIRED ACTIVITIES FOR ALL MEDICATION REVIEW SERVICES IN COMMUNITY PHARMACY
When community pharmacists choose to deliver medication review services, all three types of medication review services must be
- Provided by an authorized pharmacist or pharmacy student under the supervision of an authorized pharmacist.
- Provided as a one-on-one, in-person appointment (and not by telephone or any other electronic means),
- Provided in a suitable area that the patient accepts as respectful of their right to privacy, and
- Provided and documented in accordance with the specific requirements of this policy.
>> For details on required activities for each service type, see MR-S Required Activities, MR-PC Required Activities, and MR-F Required Activities.
Documenting medication review services that are not eligible for reimbursement
Pharmacists who conduct a medication review service for a patient who does not meet the PharmaCare eligibility requirements are encouraged to create a record of service in PharmaNet.
Use the Medication Review - Non-Benefit PIN 99000504 and, in the SIG field, enter the 10-digit phone number of the pharmacy where the service took place to record the service.
The claim will not be paid, but the patient’s PharmaNet record will indicate to other healthcare professionals that a medication review is available.
Policies and required activities for each medication review service
MEDICATION REVIEW—STANDARD (MR-S)
Required activities
For an MR-S to be eligible for PharmaCare reimbursement, the following activities must be carried out and their results documented in each of the required form(s).
|
Required Activity |
Where to document the activity results |
|
|---|---|---|
|
1 |
Confirm the patient meets all the criteria in 1. Determining patient eligibility, under Policies applicable to medication review services in communiy pharmacy. |
|
|
2 |
If the patient meets all eligibility requirements, document the patient information gathered in Step 1 above. |
|
|
3 |
Document the clinical need(s)–as listed under 1–Clinical need |
|
|
4 |
Collect and document information about patient medical issues such as known allergies and reactions. Information is collected from multiple sources including but not limited to:
|
|
|
5 |
Collect and document all pertinent information about the patient's current and recently discontinued medications (including prescription medications, non-prescription medications, and natural health products). Collect information from:
Determine whether the patient is currently taking each medication and how they are taking it. Document any clinically relevant medications the patient is no longer taking. |
|
|
6 |
Discuss, review, and document the details of each medication the patient is currently taking with the patient or their legal representative, including
|
|
|
7 |
Document all information relevant to continuity of care (e.g., details about decisions, evaluations, plans of action, and other directions or observations). Note: If a drug therapy problem is identified during an MR-S, the pharmacist is professionally responsible for taking action by working to resolve the issue or by referring the patient to an appropriate healthcare professional. If the pharmacist takes action to resolve the issue and completes one or more DTP forms, a claim for an MR-PC may be submitted instead of a claim for an MR-S. For MR‑PC required activity details, see MR-PC–Required Activities. |
|
|
8 |
Ensure all forms are fully completed, including the name and Registration ID of the pharmacist, and the contact information for the pharmacy, providing the service (to enable healthcare professionals to request the patient’s information). |
|
|
9 |
Obtain signature of patient or their legal representative in the Patient Acknowledgement section of the BPMH. If someone else is acting on the patient’s behalf, obtain documentation of that person’s right to act as the patient’s legal representative. Retain the signed original for your records. |
|
|
10 |
Provide a copy of the completed and signed Patient section of the BPMH to the patient or their legal representative. It is not necessary to provide the BPMH Health Care Professionals section to the patient. It is designed for use by clinicians only. |
|
|
11 |
Store all documents together for future reference. (For details, see PharmaCare Policy Manual, Section 10–Audit). |
|
|
12 |
Submit the medication review service claim on the date of service delivery, using the appropriate PIN. This ensures other pharmacies know that you have delivered the service to the patient and makes the clinical information available to other health care providers in a timely fashion. >> See Submitting Claims for the appropriate PIN and data entry instructions. |
|
|
13 |
When you receive a request for medication review information from a healthcare provider within the patient’s circle of care,
|
|
Required documentation
To support your claim for an MR-S service, retain the following documentation in a manner accessible for audit:
- Completed BPMH original, signed and dated by patient or their legal representative
- If applicable, documentation of another person’s right to act as the patient’s legal representative
- A written record of any requests for a copy of a patient’s BPMH (Word, 282KB)
Claims for payment
For an eligible patient, the pharmacy can submit a claim to PharmaCare for a $60 MR-S fee.
The claim must be submitted on PharmaNet on the date the medication review service is provided to the patient.
Submit the claim using the appropriate PIN and the College Registration Identification (Reg ID) of the pharmacist who provided the service to the patient.
The pharmacy must enter the 10-digit pharmacy phone number in the first 20 spaces and in front of any other information that appears in the SIG field on the patient’s PharmaNet profile to facilitate continuity of care and sharing of the BPMH (Word, 282KB) within the circle of care.
>> For details, see Submitting claims for payment.
>> For information on claim limits, see Policies applicable to medication review services, 4.Claiming medication review services fees.
MEDICATION REVIEW—PHARMACIST CONSULTATION (MR-PC)
Required activities
For an MR-PC to be eligible for PharmaCare reimbursement, the following activities must be carried out and the results documented in each of the required form(s):
|
Required Activity |
Where to document the activity results |
|
|---|---|---|
|
1 |
Ensure the patient meets the criteria for an MR-PC. The patient must
|
|
|
2 |
If the patient meets the eligibility requirements, document patient information gathered in Step 1 above. |
Patient section of
|
|
3 |
Document the clinical need(s)—as listed under 1. Clinical need—that are the reason(s) for providing the service. |
|
|
4 |
Collect and document information about patient medical issues such as known allergies and reactions. Information is collected from multiple sources including but not limited to:
|
|
|
5 |
Collect and document all pertinent information about the patient’s current and recently discontinued medications (including prescription medications, non-prescription medications and natural health products). Collect information from:
Determine whether the patient is currently taking each medication and how they are taking it. Document any clinically relevant medications the patient is no longer taking. |
|
|
6 |
Discuss, review, and document the details of each medication the patient is currently taking with the patient or their legal representative including
|
|
|
7 |
Document all information relevant to continuity of care (e.g., details about decisions, evaluations, plans of action, and other directions or observations). |
|
|
8 |
Document the identification of and actions taken/to be taken to resolve a minimum of one DTP. Work with the patient to:
Document all DTP-related decisions, plans, and actions decided upon during the appointment. Notify (and, if necessary, collaborate with) the most responsible physician or other prescriber about the DTP, care plan, and results achieved. |
|
|
9 |
Ensure all forms are fully completed, including the name and Registration ID of the pharmacist, and the contact information for the pharmacy, providing the service (to enable healthcare professionals to request the patient’s information). |
Page headers of:
|
|
10 |
Obtain signature of patient or their legal representative in the Patient Acknowledgement section of the BPMH. If someone else is acting on the patient’s behalf, obtain documentation of that person’s right to act as the patient’s legal representative. Retain the signed original for your records. |
|
|
11 |
Provide a copy of the completed and signed Patient section of the BPMH to the patient or their legal representative. |
|
|
12 |
Store all documents together for future reference. (For details, see PharmaCare Policy Manual, Section 10—Audit). |
|
|
13 |
Submit the medication review service claim on the date of service delivery, using the appropriate PIN. This ensures other pharmacies know that you have delivered the service to the patient and makes the clinical information available to other health care providers in a timely fashion. >> See Submitting Claims for the appropriate PIN and data entry instructions. |
|
|
14 |
When you receive a request for medication review information from a healthcare provider within the patient’s circle of care,
|
|
Required documentation
To support your claim for an MR-PC service, retain the following documentation in a manner accessible for audit:
- Completed BPMH original, signed and dated by patient or their legal representative
- A separate DTP form for each DTP
- If applicable, documentation of another person’s right to act as the patient’s legal representative
- If applicable, a written record of any request for a copy of a patient’s BPMH and/or DTP form(s)
Claims for payment
For eligible patients, the pharmacy can submit a claim to PharmaCare for a $70 MR-PC fee.
If, during the MR-PC, a DTP has been resolved by an action that has a separately defined PharmaCare service fee (e.g., administration of injections and/or adaptations of prescriptions), the pharmacy may submit the claims as usual, but will be reimbursed to a maximum of $70.
The claim must be submitted on PharmaNet on the date the medication review service is provided to the patient.
This ensures other pharmacies know that you have delivered the service to the patient and makes the clinical information available to other health care providers in a timely fashion.
Submit the claim using the appropriate PIN and the College Registration Identification (Reg ID) of the pharmacist who provided the service to the patient.
The pharmacy must enter the 10-digit pharmacy phone number in the first 20 spaces and in front of any other information that appears in the SIG field on the patient’s PharmaNet profile to facilitate continuity of care and sharing of the BPMH and, if applicable, DTP Form(s) within the circle of care.
>> For details, see Submitting claims for payment.
>> For information on general claim limits, see Policies applicable to medication review services in community pharmacy, 4–Claiming medication review services fees.
MEDICATION REVIEW—FOLLOW-UP (MR-F)
Required activities
For an MR-F to be eligible for PharmaCare reimbursement the following activities must be carried out and their results documented in each of the required form(s):
|
Required Activity |
Where to document the activity results |
|
|---|---|---|
|
1 |
Ensure the patient meets the criteria for an MR-F. The patient must
AND
|
|
|
2 |
If the patient meets all eligibility requirements, document patient information gathered in Step 1 above. |
|
|
3 |
Document the reason(s) for providing the MR-F service: that is, patients must have a clinical need (as listed under 1. Determining patient eligibility-clinical need) that requires the following:
|
|
|
4 |
If appropriate, review and update information about patient medical issues such as known allergies and reactions. Information is collected from multiple sources including but not limited to:
|
|
|
5 |
If the service is a follow-up due to a subsequent medication change (i.e., a change in medication that is entered on PharmaNet):
|
|
|
6 |
If the service is a follow-up to implement and/or evaluate progress towards resolving the patient’s DTP(s):
|
|
|
7 |
Document all information relevant to continuity of care (e.g., details about decisions, evaluations, plans of action, and other directions or observations). |
|
|
8 |
Ensure all forms are fully completed, including the name and Registration ID of the pharmacist, and the contact information for the pharmacy, providing the service (to enable healthcare professionals to request the patient’s information). |
Page headers of:
|
|
9 |
Obtain signature of patient or their legal representative in the Patient Acknowledgement section of the BPMH. If someone else is acting on the patient’s behalf, obtain documentation of that person’s right to act as the patient’s legal representative. Retain the signed original for your records. |
|
|
10 |
Provide a copy of the new, completed and signed Patient section of the BPMH to the patient or their legal representative. |
|
|
11 |
Store all documents together for future reference. (For details, see PharmaCare Policy Manual, Section 10–Audit). |
|
|
12 |
Submit the medication review service claim on the date of service delivery, using the appropriate PIN. This ensures other pharmacies know that you have delivered the service to the patient and makes the clinical information available to other health care providers in a timely fashion. >> See Submitting Claims for the appropriate PIN and data entry instructions. |
|
|
13 |
When you receive a request for medication review information from a healthcare provider within the patient’s circle of care,
|
|
Required documentation
To support your claim for an MR-F service, retain the following documentation in a manner accessible for audit:
- New BPMH-Patient Information section, original signed by the patient or their legal representative
- New or updated version of the BPMH-Health Care Professionals section,
- If applicable, a new or updated DTP form for each DTP
- If applicable, documentation of another person’s right to act as the patient’s legal representative
- If applicable, a written record of any request for a copy of a patient’s BPMH and/or DTP forms
- If the original MR-S or MR-PC service was provided at another pharmacy, the pharmacy providing the MR-F service must obtain a copy of the PharmaCare-required documentation for the patient’s most recent MR-S or MR-PC.
- If the original medication review was provided at a PCN, the pharmacy providing the MR-F service must obtain a copy of the most recent MR-PCN documentation from the PCN.
- If information is missing from the previous pharmacy’s or PCN's documentation, the current pharmacy should ensure that all information required for the current MR-F is obtained, documented and retained in their records.
Claims for payment
For eligible patients, the pharmacist can submit a claim to PharmaCare for a $15 MR-F fee.
Either an MR-S, MR-PC or an MR-PCN must have been claimed for the patient within the previous year.
A maximum of four MR-F claims can be made in the 12-month period following the MR-S, MR-PC or MR-PCN.
For information on general claim limits, see Policies applicable to medication review services in community pharmacy, 4. Claiming medication review services fees.
The claim must be submitted on PharmaNet on the date of the medication review service.
This ensures other pharmacies know that you have delivered the service to the patient and makes the clinical information available to other health care providers in a timely fashion.
Submit the claim using the appropriate PIN and the College Registration Identification (Reg ID) of the pharmacist who provided the service to the patient.
The pharmacy must enter the 10-digit pharmacy phone number in the first 20 spaces and in front of any other information that appears in the SIG field on the patient’s PharmaNet profile to facilitate continuity of care and sharing of the BPMH and, if applicable, DTP Form(s) within the circle of care.
>> For details, see Submitting claims for payment.
MEDICATION REVIEW—PRIMARY CARE NETWORK (MR-PCN)
Patient Eligibility
Health care practitioners in the Primary Care Network (PCN) will refer patients to a pharmacist working within the PCN. Generally, these patients will have complex medical conditions, but they may not meet the requirements for medication reviews in the community, with respect to qualifying medications, non-qualifying medications, and clinical need.
Allowable number of MR-PCNs in a year
There is no maximum number of allowable MR-PCNs in a given year. However, if a patient has received an MR-PCN, they will not be eligible for a PharmaCare-paid community medication review (MR-S or MR-PC) for the next six months. Patients may still receive up to four follow-up medication reviews (MR-F) in community after an MR-PCN, within 12 months.
Pharmacists in PCNs aren't required to use the same documentation as in community pharmacies, such as the PharmaCare Best Possible Medication History (BPMH) form or the Drug Therapy Problem (DTP) form. A community pharmacist providing a follow-up medication review (MR-F) to a client whose original medication review was provided by a pharmacist in a PCN must obtain the most recent MR-PCN documentation. As per usual MR-F procedure, the pharmacist is required to complete both the BPMH patient and the Health Care Professionals sections.
Submitting PharmaNet records
The pharmacists in the PCN will use the MR-PCN PIN to submit a $0 claim on PharmaNet after providing a medication review. Primary Care pharmacists should refer to PCN documentation for this process according to their specific PCN. Since PCNs are enabled via a partnership between regional health authorities and divisions of family practice, claims for MR-PCNs will adjudicate to $0 as medication reviews are included in the services provided by pharmacists in the PCN.
Submitting the MR-PCN PIN on PharmaNet allows for documenting the patient's care by the pharmacist in the PCN and prevents duplicate medication reviews from being provided in community pharmacies (e.g., providing an MR-S within six months after a patient has received an MR-PCN).
Procedures
Claims for medication review services must be submitted on PharmaNet on the date of the medication review, using the appropriate PIN, as shown below.
The PIN and the payment amount for each service are as follows:
| PIN | Description | Payment Amount |
|---|---|---|
| 99000501 | Medication Review Standard (MR-S) | $60.00 |
| 99000502 | Medication Review Pharmacist Consultation (MR-PC) | $70.00 |
| 99000503 | Medication Review Follow-Up (MR-F) | $15.00 |
| 99000505 | Medication Review Primary Care Network (MR-PCN) | $0.00 |
To submit a claim for a medication review service:
- In the Days Supply field, enter 1.
- In the Quantity field, enter 1.
- In the Drug Cost field, enter 0.
Entering zero in the Drug Cost field ensures the fee does not inadvertently appear on the patient's receipt. - In the DIN/PIN field, enter the appropriate PIN.
- In the SIG field, in the first 20 spaces in the field and in front of any other information that appears in the field, enter the 10-digit phone number (including area code) of the pharmacy where the service took place. Other healthcare professionals will use this number to contact you to request patient information.
If the pharmacy phone number is not entered in the first 20 characters of the SIG field, the claim will not be reimbursed. - In the Prescriber ID field, enter the College Registration Identification (Reg ID) of the pharmacist who provided the service to the patient.
Consult your software vendor to determine any other requirements for payment reconciliation.
PharmaNet response code for medication review service claims
Claims for medication review services are processed for payment in monthly batches rather than in real-time. When a claim for a medication review service is submitted, PharmaNet returns one of several “rejection” responses (e.g., CD - patient not entitled to drug claimed). These adjudication messages from PharmaNet can be ignored.
Do not reverse or re-submit claims in response to adjudication messages. If the data has been entered correctly in the requested fields, the claims will be processed for payment.
Reconciling payments
Please call the PharmaCare Help Desk about specific claims. The PharmaCare Help Desk has access to payment and claim details and can fax or mail these details (with patient identifiers removed).
Audit
All claims to PharmaCare are subject to audit and any amount associated with a disallowed claim will be recovered.
>> For information on PharmaCare audit policies, see Section 10–Audit of this manual.
Medication Review Services Forms
Best Possible Medication History (BPMH)
|
Purpose |
The purpose of the Best Possible Medication History (BPMH) (Word, 282KB) is to
The BPMH includes two sections: the Patient section and the Health Care Professionals section. The Patient section of the BPMH is a comprehensive list of all prescription medications, non-prescription medications, and natural health products the patient is currently taking on a regular or “as needed” basis.
The Health Care Professionals section of the BPMH provides a professional summary of information collected during the review suitable for sharing with other healthcare professionals.
|
|---|---|
|
When to complete form |
For every MR-S or MR-PC appointment for which a claim will be submitted to PharmaCare, complete a new BPMH-Patient section and a new BPMH-Health Care Professionals section For every MR-F appointment for which a claim will be submitted to PharmaCare, complete a new BPMH-Patient section and a new or updated BPMH-Health Care Professionals section. MR-PCNs use their own documentation. |
|
Form contents |
All content (i.e., text, fields, and field labels) included in the Best Possible Medication History template is mandatory and must be included in any pharmacy-created forms. See If you are creating your own form(s). |
|
Notes on completing the form |
All fields on all pages must be completed unless otherwise indicated. See the BPMH template for the form fields. IS IT LEGIBLE? The intent of the BPMH Patient Section is to give the patient (or their family or caregiver) a clear, written record of your discussion. To make sure your directions and comments are easy for the patient to read, use the tips below. Tips for clarity:
Ensure that the patient or their legal representative signs and dates the Patient Acknowledgement section of the BPMH. On every page of the form, include the
If the service was delivered by a pharmacy student or intern, provide the name of the pharmacist who supervised the session. If the appointment is a follow-up and the service is delivered by a different pharmacist, add the pharmacist name and Reg ID after the original pharmacist’s ID:
Optional fields include “special instructions.” Complete if applicable. Complete all fields related to clinically relevant medications that have been stopped, if the information is available. If the patient is taking more than eight medications, add additional rows to the Medications I Take and Current Medications sections as necessary, or complete additional forms. |
Drug Therapy Problem (DTP) form
|
Purpose |
The Drug Therapy Problem form is a record of all information associated with the identification, resolution, follow-up care, and communication for a DTP identified during a Medication Review—Pharmacist Consultation service appointment. This form may be shared with healthcare professionals within the patient’s circle of care at the pharmacist’s discretion. |
|---|---|
|
When to complete form |
A form must be completed whenever a DTP has been identified and resolved. A separate form must be completed for each DTP. For every MR-PC appointment for which a claim will be submitted to PharmaCare, complete a DTP form in addition to the BPMH-Patient section and BPMH-Health Care Professionals section. If applicable, for every MR-F appointment, when implementing and/or evaluating progress towards resolving the patient’s DTP, for which a claim will be submitted to PharmaCare, update each previous DTP form with new information (or generate a new one for each DTP) in addition to completing a new BPMH-Patient section and a new or updated BPMH-Health Care Professionals section. |
|
Form contents |
Pharmacists may design their own version of the form; see If you are creating your own form(s) for requirements. All content (i.e., text, fields and field labels) included in the Drug Therapy Problem (Word, 227KB) form template is mandatory and must be included in any pharmacy-created forms. See If you are creating your own form(s). |
|
Notes on completing the form |
All fields on all pages must be completed unless indicated otherwise; see the DTP form template for the form fields. If a form is illegible, the associated claim will be subject to recovery. On every page of the form, include the
If the service was delivered by a pharmacy student or intern, provide the name of the pharmacist who supervised the session. If the appointment is a follow-up and the service is delivered by a different pharmacist, add the pharmacist’s name, and Reg ID after the initial pharmacist’s ID:
Optional fields include “notification.” Complete if applicable. |
Best Possible Medication History Worksheet (BPMH Worksheet)
|
Purpose |
The Best Possible Medication History Worksheet (Word, 135KB) is an optional form that pharmacists can use to gather, record, and review the patient’s medication information before the medication review appointment. The Worksheet complies with all requirements for pharmacy printing of the PharmaNet Medication Reconciliation Report. |
|---|---|
|
When to complete form |
This form may be used as a starting point for gathering information before a medication review service appointment. Use of this form is optional. |
|
Form contents |
The contents of this form are found in the Best Possible Medication History Worksheet (Word, 135KB) template. Pharmacists may design their own version of the form; see If you are creating your own form(s) for requirements. |
|
Notes on completing the form |
N/A |
If you are creating your own form(s)
PharmaCare provides form templates that contain the minimum documentation requirements for claiming a fee for a medication review service from PharmaCare.
Any pharmacy that chooses to create their own versions of the medication review services forms must ensure that these minimum requirements are met; that is, each form must contain all the text and fields in the PharmaCare templates.
The wording of the text and field labels must not be changed.
Claims for medication review services will be reimbursed only when the forms contain all required text, fields, and field labels. If the forms do not meet these minimum requirements, claims will be subject to recovery.
Section 8.9 Tools and Resources
Form templates:
Section 8.10 – Pharmacist Administration of Drugs and Vaccines
General policy description
Pharmacies can claim a fee for administering most injectable drugs and vaccines to patients in a pharmacy.
Administering drugs and vaccines (other than publicly funded vaccines)
As of October 14, 2022, pharmacists can administer injections of all drugs, including Schedule IA drugs, but excluding allergy serums and substances for cosmetic use.
PharmaCare pays pharmacies a fee of $11.41 for administering drugs and non-publicly funded vaccines by injection (“drug administration”). The fee is claimed when a pharmacist administers a drug or vaccine by injection to a B.C. resident. An original prescription from an authorized prescriber, or a transferred prescription, is needed for pharmacists to administer Schedule II drugs (e.g., vitamin B12, dimenhydrinate) and claim the fee.
When administering drugs and non-publicly funded vaccines by injection (with the exceptions below), pharmacies must use the PIN for PharmaCare payment and may not bill the patient in addition to, or instead of, claiming the PharmaCare drug administration fee.
The PharmaCare drug administration fee cannot be claimed for administering vaccines for the indication of travel, insulin, and products designed for patient self-injection (e.g., auto-injector or pen cartridge), such as glucagon-like peptide-1 receptor agonists (GLP-1) and low molecular weight heparins (LMWH).
However, vaccines may have more than one indication. If a vaccine is administered outside of the publicly funded criteria and is for an indication other than travel, the drug administration fee of $11.41 must be claimed rather than charging the patient an administration fee.
Multi-dose vials
For multi-dose vials, the drug administration fee may be claimed for each injection. Because multi-dose vials are dispensed once, only one dispensing fee can be claimed.
Multiple daily injections
To claim multiple injections for one patient on the same day, enter the PharmaNet intervention code UF. The pharmacy can claim the administration fee for each injection; however, PharmaCare payments for iOAT injections are limited to 4 per patient per day.
Pre-filled syringes
Pre-filled syringes intended to be administered by a health care professional are eligible for the drug administration fee. See the product monograph for details on administration. Examples of administration instructions include "for administration by a health care provider" or "drug is intended for patient self-injection". The product monograph may also provide instructions for patient self-injection; the administration fee must not be claimed for products intended for patient self-injection.
Administering publicly funded vaccines
Pharmacies can elect to participate in providing publicly funded vaccinations. B.C. residents who meet the eligibility criteria set by the BC Immunization Manual receive publicly funded vaccines at no cost. The cost of the vaccine product is paid for by the provincial and/or federal government. PharmaCare pays pharmacies a fee for administering a dose of a publicly funded vaccine when the vaccine is:
- given to an eligible B.C. resident (including those covered by PharmaCare Plan B (Long-term Care)
- administered by injection (or intranasally in the case of spray flu vaccine), and
- administered by:
- an authorized pharmacist, or other authorized regulated practitioner (e.g. registered nurse), or
- for COVID-19 and influenza vaccines only, a pharmacy student under the supervision of a full pharmacist certified to administer injections, or a medical student under the supervision of a licensed practitioner (e.g., pharmacist)
The vaccine administration fee is $12.10 for all publicly funded vaccines including COVID-19 vaccines. (The COVID-19 vaccine fee was reduced from $18 to $12.10, as announced in the PharmaCare Newsletter.)
The maximum amount PharmaCare reimburses a pharmacy for any combination of medication reviews, clinical services (e.g., prescription adaptation), and drug and vaccine administrations for the same patient, on the same day is $78.00.
Publicly funded vaccination records must be entered in PharmaNet on the day the vaccination is administered.
Patient eligibility for publicly funded vaccines
Eligible B.C. residents
B.C. residents who meet the eligibility criteria set by the BC Centre for Disease Control (BCCDC) receive publicly funded vaccines at no cost.
For information on eligibility criteria, indications, contraindications and dosage for publicly funded vaccines, see the BCCDC Immunization Manual Part 4—Biological Products. This information is also available in patient-friendly language on Immunize BC’s Vaccines by Disease page.
Patients who meet BCCDC eligibility criteria but who are not B.C. residents
PharmaCare does not pay a fee to pharmacies for administering vaccines to patients who are not B.C. residents.
Pharmacists cannot charge a fee for administering a vaccine to Canadians who meet the BCCDC eligibility criteria.
Please refer all patients who meet the BCCDC eligibility criteria—but who are not B.C. residents—to a local health unit. The health unit will determine if they are eligible for free vaccinations.
Find local health clinics with Immunize BC’s Health Unit Finder.
Patients who do not meet the BCCDC eligibility criteria
Patients who do not meet BCCDC eligibility criteria cannot receive vaccines from the public supply.
Pharmacists who wish to provide vaccines to ineligible patients must obtain the vaccine from a private supplier and can charge the patient directly for the product. Administration of private supply vaccines (with the exception of travel vaccines) is paid for by PharmaCare and cannot be charged to the patient.
Vaccine records
As required by section 27(3) of the Pharmaceutical Services Act, pharmacists are responsible for entering all relevant information about vaccine administration in PharmaNet. This includes providing identifying information about the pharmacist who administered the vaccine for the purposes of professional practice review and liability.
Publicly funded influenza and COVID-19 vaccinations are entered in ImmsBC only, not in PharmaNet for:
- Publicly funded influenza vaccinations administered on or after October 1, 2022, and
- COVID-19 vaccinations administered on or after April 1, 2022
These vaccinations will not be visible in a patient’s PharmaNet dispense history. Pharmacists, physicians and other healthcare professionals can view a patient’s record of COVID-19 and influenza vaccinations in ImmsBC or CareConnect.
Information entered in ImmsBC for COVID-19 and influenza vaccinations is automatically transferred to PharmaNet and so must comply with the same standards as all PharmaNet entries. This information in PharmaNet is used to pay pharmacies the vaccination administration fee and is not accessible for clinical purposes.
Pharmacists are reminded that, when a vaccination is entered in ImmsBC, a drug use evaluation (DUE) will not automatically occur.
Information about publicly funded vaccinations must be entered in the appropriate system: ImmsBC for COVID-19 and public-supply influenza vaccines, and PharmaNet for private-supply influenza vaccines and all other vaccines administered in the pharmacy.
Procedures for pharmacists
Submitting a claim for administering drugs and non-publicly funded vaccines
Submit the claim for the drug or non-publicly funded vaccine separately from the administration fee, using the appropriate DIN (or PIN for flu vaccines that are the same as a public product – see ‘Submitting a claim when a private product is also available as public supply’ below).Enter PIN 66128366 in PharmaNet for the administration fee
- In the SIG field, enter the DIN (or PIN), generic drug name, route and administration site (RIGHT or LEFT or BILATERAL or ABDOMEN), e.g., 0273497_Vitamin B12_IM_LEFT
- In the Quantity field, enter 1
- Enter your College of Pharmacists of BC ID in the prescriber ID field
- Do not enter a drug cost or fee
- Submit the claim on the same day that the vaccine or drug is administered.
Submitting a claim for private-supply vaccines that are also available as public supply
During some influenza vaccine seasons, publicly funded products are also available as private supply. When a flu product is uniquely a private supply product, enter the DIN in PharmaNet. For products that have both a private and public supply, enter the private supply using the PIN in PharmaNet, with the pharmacist CPBC ID in the Prescriber ID field. Do not record these private supply vaccinations in ImmsBC.
These private-supply vaccinations are eligible for an administration fee of $11.41.
Submitting a claim for administering publicly funded vaccines (except COVID-19 and influenza)
Enter a publicly funded vaccination (other than influenza or COVID-19) by recording the PIN in PharmaNet on the day it is administered. For COVID-19 and influenza vaccinations, see below ‘Submitting a claim for publicly funded COVID-19 or influenza vaccinations’.
Pharmacists should follow these steps:
- Confirm that the patient is eligible to receive a publicly funded vaccination
- Obtain informed consent (PDF, 329KB)
- Provide the patient with a record of immunization with details of the vaccination
When recording the vaccination in PharmaNet, in the Directions for Use field (the SIG field), enter the lot number and vaccination site (either RIGHT or LEFT) separated by an underscore _. If entering a claim for the FluMist nasal spray, enter lot number and BILATERAL, separated by an underscore. This ensures a complete record in the Provincial Immunization Registry (PIR). A record of the vaccine lot number is required in case of a rare event such as a vaccine recall or an adverse event following immunization (AEFI).
Do not enter personally identifiable information in the SIG field, as the entire contents of the SIG field will be included in the PIR.
Submitting a claim for administering COVID-19 and influenza vaccines
- For COVID-19 vaccinations administered on or after April 1, 2022, and influenza vaccinations administered on or after October 1, 2022, only enter information in ImmsBC (not PharmaNet).
- Information entered in ImmsBC is automatically transferred to PharmaNet for fee payment to pharmacies, and will also be transferred to the PIR to complete the patient's immunization history (for clinical purposes).
While the risk of adverse side effects is low, pharmacists are reminded to check a patient’s allergies and drug history prior to administering a vaccine. If a patient has a reaction to the vaccine, record this in ImmsBC.
For claims that are not entered in PharmaNet through the local pharmacy system (i.e., COVID-19 and flu), pharmacies will not be able to reconcile monthly vaccine payments using their local system; they will need to use the reporting function available in ImmsBC. Pharmacies can request a Pharmacy Remittance Advice Form from Health Insurance BC, which shows vaccine administrations and fees entered in ImmsBC. If pharmacies want a breakdown to the individual record level, they will need to request the Service Claim Adjudication Outcome report from the PharmaCare Help Desk.
Maximum claims
The maximum amount PharmaCare will pay a pharmacy for any combination of medication administrations, medication reviews and clinical services (such as prescription adaptations) is $78.00 per patient, per day.
There is no daily limit on the number of drug administrations (i.e., injections and intranasal vaccines) to claim the drug administration fee, except for the administration of injectable opioid agonist treatment (iOAT), which is limited to 4 injections per patient, per day.
Example: A pharmacy will be paid for the administration of iOAT up to 4 injections per day, plus other drugs or vaccines or services, to the maximum of $78.00 per patient per day.
Records of COVID-19 vaccines administered between December 1, 2021 and March 31, 2022
For records of COVID-19 vaccines administered in community pharmacies between December 1, 2021, and March 31, 2022, when a vaccine record exists in both ImmsBC and in the PharmaNet dispense history, the record in ImmsBC is considered the source of authority for purposes of professional practice review, liability and clinical record.
Non-pharmacists were required to enter the note “IMMSBC” in the PharmaNet SIG field to indicate that the ImmsBC application has the complete record of the vaccination. This also identifies the person who administered the vaccine.
Section 8.10 Tools and Resources
- Drug administration fee webpage
- Publicly funded vaccines webpage
- Find local health clinics with Immunize BC’s Health Unit Finder.
- BCCDC Immunization Manual Part 4—Biological Products
- Immunize BC’s Vaccines by Disease
- How to claim PharmaCare fees for pharmacy services (PDF, 281KB)
- Drug administration fee flowchart (PDF, 50KB)
Section 8.11 – Rural Incentive Program
General Policy Description
Rural pharmacies that meet certain criteria can apply to receive a subsidy for each claim submitted for any month in which the volume of claims submitted and paid (fully or partially) by PharmaCare is below a specific threshold. Payments are made directly to the pharmacy by PharmaCare.
Policy Details
Program overview
Under the program, rural pharmacies receive a subsidy for each claim submitted if the volume of claims submitted and paid (fully or partially) by PharmaCare during the month is below a specific threshold.
Payments are made directly to the pharmacy by PharmaCare on a monthly basis.
Pharmacies do not have to change the way in which claims are submitted.
Qualifying criteria
The enhanced program is extended to rural community pharmacies and telepharmacies if the:
- Pharmacy applies for the program; and
- Pharmacy is the only pharmacy in the community; and
- Next nearest pharmacy is more than a 25 km driving distance away; or
- Next nearest pharmacy requires paid ferry travel to access it; and
- Number of PharmaCare claims submitted by the pharmacy does not exceed 1,700 per month
Telepharmacies that receive operational subsidies from a Health Authority are not eligible for the program.
Subsidies
Pharmacies with lower monthly claim volumes receive a larger subsidy for each claim.
If PharmaCare‐paid claims for a particular month exceed 1,700, no subsidy is paid.
The subsidies, calculated and paid monthly, are based on a sliding scale:
- The maximum is $10.50 per claim.
With each additional claim made within a month, the per-claim amount for the month decreases. - The minimum subsidy is $3.00 per claim.
For example, a pharmacy that submits 425 PharmaCare‐paid claims over the course of a month will receive a subsidy of $8.63 per claim. A pharmacy that submits 1,700 PharmaCare‐paid claims in a month will receive a subsidy of $3.00 per claim.
Payment limits
Payments cannot be made for partial months.
New pharmacies become eligible for the subsidy the month following the month in which their application is accepted.
Procedures
Complete the HLTH 5384 - Application for the Rural Incentive Program (PDF, 514KB) or contact the PharmaCare Help Desk to request a form.
Section 8.11 Tools and Resources
Section 8.12 – Payments to Providers
General Policy Description
Each week, PharmaCare processes payments to pharmacies and device providers and remits the payments by Electronic Funds Transfer (EFT).
Policy Details
Applying for Electronic Funds Transfer (EFT)
Payments to providers are deposited electronically into a designated account at a financial institution as specified on the Direct Deposit Application form. This form must be mailed, along with an original void cheque, to:
PharmaCare Information Support
PharmaCare
P.O. Box 9655 Stn Prov. Govt.
Victoria B.C. V8W 9P2
Providers must also submit a Direct Deposit Application form to PharmaCare when:
- The EFT bank account changes, or
- Any other information about the EFT bank transaction changes.
Direct Deposit Application forms are available upon request from PharmaCare Information Support.
Only Parts 1, 2 and 3 of the Direct Deposit Application form should be completed. If an original void cheque is not attached to the form, then the “Bank or Financial Institution Verification” section in Part 2 must be completed.
A provider representative must sign in the “Supplier/Employee Signature” area of Part 3 of the Direct Deposit Application form. If the form is not signed, Health Insurance BC will return it unprocessed.
Information about payments
The first electronic deposit is made approximately eight to 12 weeks after receipt of the Direct Deposit Application form. Ongoing payments by EFT are scheduled for deposit on the Monday following the end of each payment period.
If, for any reason, the EFT payment cannot be deposited to the bank account on file with PharmaCare, the funds are returned to the Ministry of Finance.
Changes to banking information
When a provider changes its banking information for Electronic Funds Transfer (EFT), PharmaCare must receive a completed Direct Deposit Application form and a void cheque for the new account at least eight to 12 weeks before the change takes effect. This ensures that EFT payments will not be interrupted due to the change.
The old account must be left open until a payment has been received in the new account.
Accessing provider payment data on PharmaNet
Pharmacy Remittance Advice Forms are sent only to:
- Suppliers who are not connected to PharmaNet
- Providers, dispensing physicians/clinics, or non pharmaceutical suppliers if payment adjustments have been made
All payments processed as adjustments (including Plan B capitation rates and Methadone Maintenance Program interaction fees) are included in the final payment run of each month. Providers receive payment at the end of the month following the month of service.
Payment data (except payment adjustment information) can be accessed on PharmaNet using the Daily Totals (TDT) retrieval transaction.
All Pharmacy Software Vendor (PSV) products contain the Retrieve Daily Totals feature although it may be called by a different name. Providers can consult their software user manual or call their vendor for information on using this feature.
To calculate your expected remittance amount, add the Daily Totals for a payment period.
This summary of payment for a pay period may already be provided by your PSV product or by your current accounting procedures.
The payment period is Tuesday to Monday, with payments scheduled for deposit on the following Monday. (For example, for the payment period August 4, 2009, to August 10, 2009, the day of deposit would be August 17, 2009.) A statutory holiday would delay the deposit.
Daily Totals data are available only for the current date and the preceding 45 days. If you require payment data from before that period, contact the PharmaCare Help Desk. The Help Desk can retrieve earlier data on request.
Section 8.13 – Patient Support Fees
General Policy Description
PharmaCare may occasionally announce an initiative or campaign that would benefit from pharmacists providing specific support or information to patients.
PharmaCare may pay pharmacists a fee for their support services during a campaign.
Policy Details
Payments to pharmacists
A patient support fee is a fixed fee, with the dollar value for each campaign to be determined by PharmaCare.
The fee is set up as a non-benefit Product Identification Number (PIN). A monthly payment is issued to each pharmacy.
Only one support fee may be claimed per patient per campaign.
When a patient support fee is available, the details are published on the Related Services List under Plan M (Medication Management).
Pharmacy eligibility
Any pharmacy that is a PharmaCare provider can claim the patient support fee.
Patient eligibility
PharmaCare identifies the eligible patient cohort for each campaign.
Procedures
PharmaCare determines the requirements for the patient support fee for each campaign. Instructions for claiming a patient support fee will be announced in the PharmaCare Newsletter at the beginning of each campaign.
| Current patient support fees | Amount |
|---|---|
| There are no current patient support fees | N/A |
Section 8.14 Minor Ailments and Contraception Service
Effective June 1, 2023, pharmacists in British Columbia, are enabled to prescribe for certain minor ailments and contraception through Ministerial Order 114/2023.
General Policy Description
B.C. pharmacies can submit claims to PharmaCare for providing a Minor Ailments and Contraception Service (MACS) to eligible patients. MACS can only be provided by pharmacists who have completed the PPMAC Regulatory Education module, and for the minor ailments listed in Schedule A of Ministerial Order 114/2023 or for contraception.
MACS is provided through a private in-person visit during which the pharmacist conducts a comprehensive assessment and offers clinical advice and treatment for minor ailments and/or contraception needs. The pharmacist may prescribe medications, offer non-prescription medications, make non-pharmacological recommendations, and/or advise the patient to seek medical attention from a physician or other health care professional.
This policy standardizes how PharmaCare MACS is delivered across B.C. For details on standards, limits and conditions, and other information related to diagnosing or prescribing, refer to the College of Pharmacists of BC’s Pharmacist Prescribing for Minor Ailments and Contraception (PPMAC).
Policy Details
Pharmacists should ensure they know the entire contents of this section of the PharmaCare Policy Manual before delivering and submitting claims for MACS.
1. Determining patient eligibility
Before conducting MACS for a consenting patient for which a claim will be submitted, pharmacists must ensure the patient is eligible for PharmaCare coverage of the service.
To be eligible to receive PharmaCare-covered MACS, the patient must meet all of the criteria in the table below:
| The patient must | Notes |
|---|---|
| Be a resident of B.C. | Must have a permanent address in B.C., verified by a B.C. driver’s licence, BC Services Card or other ID. |
| Have a BC Personal Health Number (PHN) | B.C. residents with a PHN are eligible, even if they are not covered by or registered with PharmaCare, such as beneficiaries of Non-Insured Health Benefits and Canadian Armed Forces. |
| Not be covered under PharmaCare Plan B (Long-term Care) | Patient is under the care of another healthcare provider. |
| Have a clinical need for service | Patient must self-identify as having a minor ailment listed under Schedule A of the Pharmacists Regulation or a contraception need, and initiate a request for MACS. |
Time interval between assessments
The minimum time interval between assessments for the same minor ailment or for contraception for the same patient is three days. For example, if a claim was submitted on Monday, the 3-day interval would be Tuesday to Thursday, and an assessment can be eligible for PharmaCare claim again for the same minor ailment or contraception for the same patient on Friday. These claims are adjudicated in monthly batches.
| Condition | PIN Description (PA – Pharmacist Assessment) (HCP – healthcare provider) |
PINs1 |
|---|---|---|
| Acne, mild | PA acne-RX | 98890001 |
| PA acne-RX other HCP | 98890002 | |
| PA acne-no RX | 98890003 | |
| PA acne-no RX other HCP | 98890004 | |
| Allergic rhinitis | PA allergy-RX | 98890005 |
| PA allergy-RX other HCP | 98890006 | |
| PA allergy-no RX | 98890007 | |
| PA allergy-no RX other HCP | 98890008 | |
Conjunctivitis
|
PA pink eye-RX | 98890009 |
| PA pink eye-RX other HCP | 98890010 | |
| PA pink eye-no RX | 98890011 | |
| PA pink eye-no RX other HCP | 98890012 | |
| Contraception | PA contraception-RX | 98890013 |
| PA contraception-RX other HCP | 98890014 | |
| PA contraception-no RX | 98890015 | |
| PA contraception-no RX other HCP | 98890016 | |
Dermatitis
|
PA dermatitis-RX | 98890017 |
| PA dermatitis-RX other HCP | 98890018 | |
| PA dermatitis-no RX | 98890019 | |
| PA dermatitis-no RX other HCP | 98890020 | |
| Dysmenorrhea | PA menstrual pain-RX | 98890021 |
| PA menstrual pain-RX other HCP | 98890022 | |
| PA menstrual pain-no RX | 98890023 | |
| PA menstrual pain-no RX other HCP | 98890024 | |
Fungal infections
|
PA fungal infx-RX | 98890029 |
| PA fungal infx-RX other HCP | 98890030 | |
| PA fungal infx-no RX | 98890031 | |
| PA fungal infx-no RX other HCP | 98890032 | |
| GERD/dyspepsia | PA GERD/dyspepsia-RX | 98890033 |
| PA GERD/dyspepsia-RX other HCP | 98890034 | |
| PA GERD/dyspepsia-no RX | 98890035 | |
| PA GERD/dyspepsia-no RX other HCP | 98890036 | |
| Headache | PA headache-RX | 98890037 |
| PA headache-RX other HCP | 98890038 | |
| PA headache-no RX | 98890039 | |
| PA headache-no RX other HCP | 98890040 | |
| Hemorrhoids | PA hemorrhoid-RX | 98890041 |
| PA hemorrhoid-RX other HCP | 98890042 | |
| PA hemorrhoid-no RX | 98890043 | |
| PA hemorrhoid-no RX other HCP | 98890044 | |
| Herpes labialis (cold sores) | PA cold sore-RX | 98890045 |
| PA cold sore-RX other HCP | 98890046 | |
| PA cold sore-no RX | 98890047 | |
| PA cold sore-no RX other HCP | 98890048 | |
| Impetigo | PA impetigo-RX | 98890049 |
| PA impetigo-RX other HCP | 98890050 | |
| PA impetigo-no RX | 98890051 | |
| PA impetigo-no RX other HCP | 98890052 | |
| Oral ulcers (canker sores, aphthous ulcers) | PA canker sore-RX | 98890053 |
| PA canker sore-RX other HCP | 98890054 | |
| PA canker sore-no RX | 98890055 | |
| PA canker sore-no RX other HCP | 98890056 | |
| Oropharyngeal candidiasis (oral thrush) | PA oral thrush-RX | 98890057 |
| PA oral thrush-RX other HCP | 98890058 | |
| PA oral thrush-no RX | 98890059 | |
| PA oral thrush-no RX other HCP | 98890060 | |
| Musculoskeletal sprains and strains | PA MSK pain-RX | 98890061 |
| PA MSK pain-RX other HCP | 98890062 | |
| PA MSK pain-no RX | 98890063 | |
| PA MSK pain-no RX other HCP | 98890064 | |
| Shingles (herpes zoster) | PA shingles-RX | 98890065 |
| PA shingles-RX other HCP | 98890066 | |
| PA shingles-no RX | 98890067 | |
| PA shingles-no RX other HCP | 98890068 | |
| Nicotine dependence | PA nicotine-RX | 98890069 |
| PA nicotine-RX other HCP | 98890070 | |
| PA nicotine-no RX | 98890071 | |
| PA nicotine-no RX other HCP | 98890072 | |
| Threadworms or pinworms | PA pinworm-RX | 98890073 |
| PA pinworm-RX other HCP | 98890074 | |
| PA pinworm-no RX | 98890075 | |
| PA pinworm-no RX other HCP | 98890076 | |
| Urinary tract infection (uncomplicated) | PA UTI-RX | 98890077 |
| PA UTI-RX other HCP | 98890078 | |
| PA UTI-no RX | 98890079 | |
| PA UTI-no RX other HCP | 98890080 | |
| Urticaria, including insect bites | PA hives/bites-RX | 98890081 |
| PA hives/bites-RX other HCP | 98890082 | |
| PA hives/bites-no RX | 98890083 | |
| PA hives/bites-no RX other HCP | 98890084 | |
| Vaginal candidiasis (yeast infection) | PA yeast infx-RX | 98890085 |
| PA yeast infx-RX other HCP | 98890086 | |
| PA yeast infx-no RX | 98890087 | |
| PA yeast infx-no RX other HCP | 98890088 |
1Refer to the submitting claims section
Minor ailment and contraception services not eligible for MACS fee
PharmaCare will not pay pharmacies the fee for MACS provided to ineligible patients or for virtual services. When providing services that are not eligible for the MACS fee, please use the appropriate non-benefit PIN shown below.
| Service description | PIN |
|---|---|
| Non-benefit minor ailment or contraception service | 98890089 |
| Virtual non-benefit minor ailment or contraception service | 98890090 |
Federally insured B.C. residents
PharmaCare will pay pharmacies for MACS provided to federally insured B.C. residents, including individuals insured under Non-Insured Health Benefits, Veteran Affairs Canada and Canadian Armed Forces, as long as their insurance does not cover a service that is the same as MACS.
| Service description | PIN |
|---|---|
| MACS for federally insured B.C. residents | 9800091 |
2. Required activities for MACS
When a pharmacist delivers MACS, they must do so in accordance with the standards, limits and conditions defined by the BC Health Professions Act Bylaws Schedule F, Part 8 – Pharmacist Diagnosing and Prescribing. In addition, the pharmacist must:
- Conduct a consultation with the patient or their legal representative*, if applicable, in person (and not by telephone or any other electronic means)
- Provide MACS in a suitable area that the patient accepts as respectful of their right to privacy, and meets their requirements for cultural safety
- Document the assessment in accordance with the requirements of this policy
For MACS to be eligible for PharmaCare reimbursement, the following activities must be carried out and documented on the MACS form:
- Inform the patient of the service, ensure their coverage eligibility and clinical eligibility, and obtain informed consent before initiating the service.
- Review the patient's PharmaNet profile.
- Determine the nature of the patient’s symptoms and assess their medical and medication history.
- Recommend appropriate treatment, which may include medication, self-care advice, and/or advising the patient to see other appropriate health care professional(s)(HCP), if necessary.
- Provide advice on how to take the prescribed or recommended medication, any potential side effects or interactions, and what to do if the symptoms do not improve.
- Establish, implement, document and inform the patient of their follow-up and monitoring plan. This may include:
- Informing the patient of the need for follow-up with the pharmacist to monitor the effectiveness and safety of treatment;
- Monitoring the patient for any adverse drug events and the patient’s response to treatment;
- Stopping drug therapy if it is not effective or the risks outweigh the benefits; and
- Informing the patient when to seek medical attention from another health care provider.
*In BC, an acceptable legal representative for a patient must be acting under a representation agreement under the Representation Agreement Act or a committee appointed under the Patients Property Act. Whenever someone is acting on a patient’s behalf, the pharmacy must retain documentation of that person’s right to act as the patient’s legal representative.
3. Documenting MACS
Pharmacies that submit MACS claims must retain complete documentation to support their claim.
Documenting MACS:
- Provides patients, caregivers, other healthcare professionals and, in rare cases, the BC Coroners Service, with accurate, complete, and current information about the assessment and recommendations
- Provides auditable proof that an eligible MACS occurred
Required documentation
Following are the minimum documentation requirements for a PharmaCare claim:
- The full name of the patient, patient phone number, and PHN
- Confirmation of informed consent
- The minor ailment of concern or contraception request
- Confirmation of PharmaNet check
- Confirmation of patient eligibility
- Patient assessment, including a description of signs and symptoms, review of relevant medical history and medications, and diagnosis. If a diagnosis is outside the scope of the pharmacist, it can be indicated as “advised to see another healthcare provider as out of scope”
- Recommendations, including:
- Whether a prescription was issued
- Whether the patient was advised to see another healthcare provider, and
- Details of prescription and/or other recommendations, including non-pharmacological or self-care strategies
- Monitoring and follow-up plan
- Notification to primary care provider, if applicable, including date and method of notification. Any notification must be on the same day the MACS was conducted.
- Pharmacy name, phone number, and address; printed name of pharmacist and licence number; pharmacist signature and date signed. The date signed must match the date of the MACS service delivery
A sample MACS form (under Tools and Resources below) is included as a reference. Pharmacies may use the template to record required information or create their own forms. Claims for MACS will be subject to recovery if the form does not include all the required information above.
Document retention and storage
Documents must be retained on paper or electronically, in the same manner as other patient records. For more information, see Provider Recordkeeping Requirements.
4. Claiming MACS fees
If MACS is provided to an eligible patient, pharmacies must not request or accept additional fees or payment from the patient or third-party payer.
To support a claim, the completed and original MACS form must be retained in a manner accessible for audit.
PharmaCare will pay pharmacies $20 for each MACS provided to an eligible patient. The MACS fee will be included in the $78 PharmaCare maximum daily limit. (See note below regarding medication reviews.)
The claim must be submitted to PharmaNet on the date the MACS is provided to the patient. The appropriate PIN and College Registration Identification (Reg ID) of the pharmacist who provided the MACS must be submitted with the claim.
The pharmacy must enter the 10-digit pharmacy phone number at the start of the SIG field to facilitate sharing of the information within the patient’s circle of care.
Medication Review Services and MACS
PharmaCare will not reimburse a medication review service claim and a MACS claim for the same patient if both are conducted on the same day.
Frequent dispensing
PharmaCare will not reimburse pharmacies for dispensing fees when they prescribe medications for daily and/or frequent dispensing.
Procedures
Submitting claims
Claims for MACS must be entered in PharmaNet on the date of the assessment, using the appropriate PIN. Contraception and each minor ailment have a set of four PINs which indicate whether the prescription is for a schedule I drug and whether the patient was advised to seek medical attention from another healthcare professional.
Use PINs with RX for assessments that resulted in a Schedule 1 drug being prescribed and the patient was not advised to seek medical attention from another healthcare professional.
Use PINs with RX other HCP for assessments that resulted in a Schedule 1 drug being prescribed and the patient was advised to seek medical attention from another healthcare professional.
Use PINs with no RX for assessments that resulted in a non-prescription recommendation and/or non-pharmacological advice and the patient was not advised to seek medical attention from another healthcare professional.
Use PINs with no RX other HCP for assessments that resulted in a non-prescription recommendation and/or non-pharmacological advice and the patient was advised to seek medical attention from another healthcare professional.
“Other HCP” would mean an escalation of care to a physician, nurse practitioner, urgent primary care centre, or emergency department. If a patient is advised to visit a physiotherapist, massage therapist, or other allied health professional for complementary care, this would not be included in reasons to use the other HCP PINs.
To submit a claim for MACS (see below for non-benefit claims):
- In the Days Supply field, enter 1.
- In the Quantity field, enter 1.
- In the Drug Cost field, enter 0.
Entering zero in the Drug Cost field ensures the fee does not appear on the patient's receipt. - In the DIN/PIN field, enter the appropriate PIN.
- In the SIG field, enter the 10-digit phone number (including area code) of the pharmacy where the service took place. Other healthcare professionals will use this number to contact you to request patient information.
If the pharmacy phone number is not entered in the first 20 characters of the SIG field, the claim will not be reimbursed. - In the Prescriber ID field, enter the College Registration Identification (Reg ID) of the pharmacist who provided the service to the patient.
Consult your software vendor to determine any other requirements for payment reconciliation.
To record a non-benefit or virtual non-benefit assessment:
Complete steps above, however for #5, enter the ailment assessed in the SIG field as well as the pharmacy phone number. We ask pharmacies to enter non-benefit assessments into PharmaNet for statistical purposes.
Prescriptions for Schedule 1 drugs resulting from MACS
Prescriptions resulting from MACS must include the same requirements of a prescription as set out by the College of Pharmacists of BC.
Please use the following procedure when submitting prescriptions for schedule 1 drugs resulting from a MACS assessment.
- In the PRACT ID Ref field, enter P1 — College of Pharmacists of BC.
- In the PRACT ID field, enter the prescribing pharmacist’s College ID.
- Include the PS intervention code.
| Code | Description |
| PS | Professional Care Service |
PharmaNet response code for MACS claims
Reconciling payments
Please call the PharmaCare Help Desk about specific claims. The PharmaCare Help Desk has access to payment and claim details and can fax or mail these details (with patient identifiers removed).
Audit
All claims to PharmaCare are subject to audit and any amount associated with a disallowed claim will be recovered.
For information on PharmaCare audit policies, see Section 10—Audit of this manual.

Resources
Section 8.15 – Rapid antigen test kit distribution
PharmaCare has ended payments to pharmacies for distribution of RAT kits. Policy 8.15 was in effect until June 30, 2025.
The last day to order RAT kits that are eligible for the PharmaCare distribution fee was May 31, 2025. Pharmacies had until June 30, 2025 to submit claims for the distribution fee for RAT kits that were:
- ordered by May 31, 2025, and
- opened for public distribution prior to June 30, 2025.
Late claims submitted after June 30 will not be reimbursed.
Distributors will continue to supply tests at no cost to pharmacies while program supplies last, though pharmacies cannot claim the distribution fee on tests ordered after May 31, 2025. All provincially supplied tests must be provided to patients free of charge. Pharmacies wishing to provide RAT kits after the provincial distribution program ends can do so through a private-pay model.
Refer to the May 2025 PharmaCare Newsletter for more details.
General policy description
Community pharmacies may claim a fee for distributing, at no charge to clients, publicly funded COVID-19 rapid antigen test (RAT) kits and instruction sheets.
Policy details
Non-pharmacy staff may hand out RAT kits and instruction sheets, but pharmacy staff must enter the PIN in PharmaNet on opening a case (see Procedures). RAT kits must be distributed at no charge to the client. They must be handed out one at a time.
RAT tests, kits, and cases are to be managed separately from any retail stock and are not for resale. Please maintain your records of RAT cases received, as these claims are subject to PharmaCare audit and recovery.
Pharmacy eligibility
Any pharmacy that is a PharmaCare provider can qualify for the RAT kit distribution fee. The client they distribute kits to does not need to be covered by PharmaCare.
Patient eligibility
Kits can be given to anyone 18 and over. They do not need to be a B.C. resident. They do not need to show ID. They may collect a kit for another person. The pharmacy does not need to collect or record their PHN.
Procedures
A case of Rapid Response® COVID-19 antigen test (RAT) kits contains about 115 kits; the amount can range between 91 to 140 kits. Pharmacies will be paid $75.00 per case distributed, regardless of the number of kits in a case. A kit includes 5 tests.
The BCCDC instruction sheet is available for printing on the BCCDC website in several languages. A pharmacy may also provide a QR code that links to the instruction sheet, if the client prefers.
Health Canada periodically extends RAT kit expiry dates. Visit Health Canada’s web page, Authorized medical devices for uses related to COVID-19 and the BC Centre for Disease Control COVID-19 website for current information.
PharmaNet instructions for claiming the RAT kit distribution fees
1. On opening a case of Artron Rapid Response® COVID-19 rapid antigen test (RAT) kits, enter the PIN: 66128338. For a case of BTNX RAT kits enter the PIN: 66128325.
2. Enter your pharmacy’s O-Med PHN instead of the patient PHN.
3. Enter Quantity as the number of cases. Do not enter the quantity as the number of tests or kits. Claims are monitored to ensure that quantities are entered correctly.
4. The claim will adjudicate as a non-benefit and will be paid monthly. Payments will appear on the Pharmacy Remittance Advice Form under the Adjustment Code “7 – Manual Payment.”
Resources
Artron RAT kit instructions: English | ASL | Arabic | Simplified Chinese | Traditional Chinese | Farsi | French | Korean | Punjabi | Spanish | Vietnamese | Tigrinya | Russian | Ukrainian
Section 8.16 – Prescribed Alternatives Witnessing Fee
The Ministry is working on a new payment system to pay pharmacies a fee for witnessing doses of prescribed alternatives (PAs).
Pharmacies can expect to receive payments in spring 2026, once the new payment system is ready to be implemented. Pharmacies will receive retroactive payments for witnessed doses of PAs recorded in PharmaNet from June 18, 2025, onwards.
General policy description
PharmaCare will pay pharmacies a fee for each eligible dose of witnessed PA medications up to a daily maximum. Fee amounts are based on the type of medication.
Pharmacists and other regulated health professionals must witness according to instructions described in the provincial policy Access to Prescribed Alternatives in British Columbia (PDF, 304KB) and the BC Centre on Substance Use (BCCSU) Clinical Bulletin: Updated Prescribed Alternatives Policy–Implications for Clinical Practice (PDF, 2.6MB).
Policy details
Eligibility
Prescribers are requested to write “SA” on all PA prescriptions (for “safer alternative”). They must not use "SA" for opioid, stimulant or benzodiazepine drugs when they are prescribed for non-PA indications.
To be eligible for a PA witness fee:
- "SA" must be written on the prescription by the prescriber*
- The drug must be a PA drug from the table below
- The drug must be ingested orally or applied topically**
*If "SA" is not written in the original prescription, and the pharmacist has confirmed with the prescriber that it is intended as PA, the pharmacist should denote it as a PA prescription by inputting the associated intervention codes.
**A fentanyl patch exchange regimen where a patient exchanges their used fentanyl patches for new fentanyl patches constitutes witnessed dosing.
PAs witnessed in a community pharmacy must be witnessed by a pharmacist.
PAs witnessed as part of a delivery or outreach model of care (i.e., delivered directly to the client) must be witnessed by a regulated health professional (e.g., pharmacist, nurse).
PAs witnessed in a clinic by a regulated health professional are not eligible for the PharmaCare witness fee.
PharmaCare will pay up to a maximum number of witness fees per client per day, as detailed on the PA drug list below.
PA witness fees are subject to audit and recovery. Refer to the PharmaCare Policy Manual, Section 10.1: Audit Policies for details.
| PA drugs eligible for a witness fee – includes all dosages | ||||
|---|---|---|---|---|
| Drug class | Chemical type | Formulation type | Witness fee amount | Daily maximum number of fees |
| Opioid | Fentanyl | Patch | $10 | 1 |
| Fentanyl | Tablet (e.g., Fentora) | $7 | 3 | |
| Hydromorphone | Capsule 12h ER | $4 | 2 | |
| Hydromorphone | Tablet | $4 | 3 | |
| Hydromorphone | Syrup | $4 | 3 | |
| Morphine | Capsule 12h ER (e.g., M-Eslon) |
$4 | 2 | |
| Morphine | Tablet ER (e.g., MS Contin) | $4 | 2 | |
| Morphine | Syrup | $4 | 3 | |
| Oxycodone | Tablet 12h ER (e.g., Oxyneo) | $4 | 2 | |
| Oxycodone | Tablet | $4 | 3 | |
| Benzodiazepine | Alprazolam, clobazam, clonazepam, diazepam, lorazepam, oxazepam | Tablet | $4 | 3 |
| Chlordiazepoxide | Capsule | $4 | 1 | |
| Lorazepam | Sublingual tablet | $4 | 3 | |
| Stimulant | Dextroamphetamine | Capsule ER (e.g., Dexedrine Spansule) | $4 | 2 |
| Dextroamphetamine | Tablet | $4 | 3 | |
| Dextroamphetamine | Capsule ER 24h (e.g., Adderall XR) | $4 | 1 | |
| Lisdexamfetamine | Capsule | $4 | 1 | |
| Methylphenidate | CSBP 40-60 (e.g, Biphentin) | $4 | 2 | |
| Methylphenidate | Tablet ER 24h (e.g., Concerta) | $4 | 1 | |
| Methylphenidate | Tablet | $4 | 3 | |
| Methylphenidate | Tablet ER (e.g., Ritalin SR) | $4 | 2 | |
Procedures
Submitting claims
Claims must be submitted on the day the PA was dispensed and witnessed. Multiple daily doses for one patient should not be processed to PharmaNet at the same time. For example, if a patient is prescribed four doses of hydromorphone daily, those four doses should not be processed all together before the patient arrives.
Claim reversals will not be paid. Claims submitted on a different date than the actual dispense will not be paid and/or will be subject to recovery.
PAs witnessed in a clinic
When dispensing PAs to be witnessed in a clinic, pharmacies must use the PharmaNet intervention code “AW – PA Sent for witnessing by other health care professional”. PharmaCare does not pay pharmacies for witnessing that takes place in a clinic.
Note: Clinics will update a patient record on PharmaNet when they witness doses; refer to TMUs in PharmaNet for IIMOAT (PDF, 166KB) for further information.
PAs witnessed in a community pharmacy or delivered to a client
Claims for all witnessed doses of PAs witnessed in a community pharmacy or delivered to a client must be notated with the PharmaNet intervention code “VS – Other outcome or intervention”.
If dispensing more than once in a day, enter both:
- VS – Other outcome or intervention, AND
- UF – Patient gave adequate explanation, Rx filled as written
The VS code must be entered for each instance of witnessed consumption of PA. For example, if hydromorphone 8 mg is witnessed three times daily at the pharmacy, there should be three claims submitted using the appropriate intervention codes.
Pharmacies are encouraged to indicate the frequency of witnessed ingestion in the SIG field.
When dispensing a PA prescription that includes both witnessed and non-witnessed doses, enter both:
- VS – Other outcome or intervention, AND
- SA – Prescribed safer alternative
Record frequency of witnessed and non-witnessed doses in brackets at the end of the SIG field (e.g., witness 3 times/day). The field limit is 80 characters only; long entries are cut short. Please use numerals ("3" instead of "three).
For instructions on how to record exclusively unwitnessed doses of PAs, refer to Dispensing exclusively unwitnessed PAs.
| Prescribed alternative scenario | Intervention code to use | |
|---|---|---|
| Witnessed | First interaction of the day: VS Subsequent interactions same day: VS and UF |
|
| Witnessed with carries | First interaction of the day: VS and SA Subsequent interactions same day: VS and UF |
|
| Not witnessed | First dispense of the day: SA Subsequent dispenses same day: SA and UF |
|
| Witnessed in a clinic | Witnessed in a clinic: AW | |
| Example 1 | ||||
|---|---|---|---|---|
| RX: Hydromorphone 8 mg tablets – 1-3 tablets q1h as needed up to 14 tablets provided daily (SA witness) | ||||
| Dispense # in day | SIG/Directions | Quantity to PNet | Intervention codes | |
| First | Take 1-3 tabs/hr as directed, max 14 tabs/day (Witness) | 3 | VS | |
| Second | Take 1-3 tabs/hr as directed, max 14 tabs/day (Witness) | 3 | VS and UF | |
| Third | Take 1-3 tabs/hr as directed, max 14 tabs/day (Witness) | 3 | VS and UF | |
| Example 2 | ||||
|---|---|---|---|---|
| RX: Hydromorphone 8 mg tablets – 1-3 tablets q1h as needed up to 14 tablets provided daily. (SA witness first dose with remainder as carry) | ||||
| Dispense # in day | SIG/Directions | Quantity to PNet | Intervention codes | |
| First | Take 1-3 tabs/hr as directed, max 14 tabs/day (Witness 1 time daily) | 14 | VS and SA | |
| Example 3 | ||||
|---|---|---|---|---|
| RX: Hydromorphone 8 mg tablets – 1-3 tablets q1h as needed up to 14 tablets provided daily.(SA witness twice daily with remainder as carry) | ||||
| Dispense # in day | SIG/Directions | Quantity to PNet | Intervention codes | |
| First | Take 1-3 tabs/hr as directed, max 14 tabs/day(Witness 2 times daily) | 7 | VS and SA | |
| Second | Take 1-3 tabs/hr as directed, max 14 tabs/day(Witness 2 times daily) | 7 | VS and UF | |
Documentation
Witness accountability logs
As of December 4, 2025, each witnessed dose of PA must be recorded in a witness accountability log to claim the PharmaCare witness fee. The log must include:
- Date and time the drug was dispensed and witnessed
- Prescription or transaction number
- Quantity of the drug witnessed
- Quantity provided as unwitnessed doses (“carries”), if any
- Total quantity dispensed
- Signature of the client
- Signature or initials of the witnessing pharmacist (if dose was witnessed in a community pharmacy) or witnessing regulated health professional (if dose was delivered on behalf of the pharmacist as part of a delivery or outreach model of care)
Any fees associated with the dispense, including the witness fee, will be recovered if the log is not filled out correctly.
The PA witness log is for PharmaCare auditing purposes and is not required for circumstances when PharmaCare does not pay any witness fees. This includes situations when:
- PAs are dispensed to a clinic for witnessing by a regulated health professional
- PA drugs are not eligible for the witness fee (i.e., drugs not listed on the PA drug list)
- Dispensing PAs where the prescriber has indicated that all doses are to be unwitnessed
Pharmacies can use existing methadone witness logs as a template with two modifications: add a column for marking the time when the PA dose was witnessed, and if deliveries are witness by a health professional other than a pharmacist, change “Pharmacist Initials” column to “Pharmacist or Health Professional Initials”.
Dispensing exclusively unwitnessed PAs
When a dispensing a PA prescription where prescriber has clearly indicated that all doses are not to be witnessed, enter the PharmaNet intervention code “SA”.
If dispensing multiple non-witnessed doses of PA in a day, enter both the “SA” intervention code as well as the “UF – Patient gave adequate explanation, Rx filled as written” code.
Resources
- Access to Prescribed Alternatives in British Columbia (PDF, 304KB)
- Clinical Bulletin: Updated Prescribed Alternatives Policy–Implications for Clinical Practice (PDF, 2.6MB)

9 - Privacy
Section 9.1 – Identifying Patients
General Policy Description
Pharmacists must take reasonable steps to identify a patient, patient’s representative, practitioner or pharmacist before providing pharmacy services.
Policy Details
Requirements for confirming identity
As per section 36 of the Pharmacy Operations and Drug Scheduling Act (PODSA) bylaws (PDF, 258KB), pharmacists “must take reasonable steps to confirm the identity of a patient, patient's representative…before providing any pharmacy service that requires accessing, using or disclosing patient personal health information.”
The College of Pharmacists of BC (CPBC) Professional Practice Policy 54–Identifying Patients and Patient Representatives in Community Pharmacy and Telepharmacy Settings (PDF, 155KB) establishes that
- Pharmacists must ensure that only one PharmaNet patient record is created and maintained for each person and that only one Personal Health Number (PHN) is assigned to each person. By viewing and confirming appropriate identification documents, duplicate PHNs and patient records can be avoided
- When a patient or patient’s representative is personally known to the pharmacist, the pharmacist may identify the patient or patient’s representative
- If the patient or patient’s representative is not known to the pharmacist, identification can be made by viewing one piece of primary identification or two pieces of secondary identification.
- When a patient or patient's representative doesn't have primary or secondary identification, the pharmacist should use their professional judgement for identification and ensure these steps are documented
The CPBC Professional Practice Policy 54–Identifying Patients and Patient Representatives in Community Pharmacy and Telepharmacy Settings (PDF, 210KB) lists these examples of primary identification:
- Driver’s licence
- Passport
- BC Services Card
- Police identity card issued by the RCMP or municipality
- Secure Certificate of Indian Status or Certificate of Indian Status
- Permanent resident card issued by the Government of Canada
The CPBC Professional Practice Policy 54 (PDF, 210KB) lists these examples of secondary identification:
- CareCard issued by the Province of B.C.
- Birth certificate
- Canadian citizenship card
- Record of landing of permanent residency
- Work/visitor/study permit issued by the Government of Canada
- Naturalization certificate
- Marriage certificate
- Change of name certificate
- Identification or discharge certificate from Global Affairs Canada or Canadian Armed Forces
Different identification requirements apply when managing patient protective words. (i.e., when applying, removing or changing a protective word on the patient’s own PharmaNet record).
Refer to Section 9.6 – Protective Words for details.
Protecting patient information from fraudulent use or identity theft
Pharmacies should report suspicious requests for pharmacy or client information to the PharmaCare Help Desk. They should refuse phone requests for such information if they cannot identify the caller.
HIBC never contacts pharmacies for pharmacist credentials or other identifying information. Such requests are often an attempt to obtain drugs illegally or commit identity theft. If someone calls a pharmacy claiming to be from PharmaCare, PharmaNet or HIBC, pharmacies should call the PharmaCare Help Desk to make sure the call is from them before engaging.
When you call the PharmaCare Help Desk, please be prepared to provide information to prove your identity. HIBC carefully screens callers to help prevent misuse of pharmacy or pharmacist information.
Tools and Resources
CPBC Professional Practice Policy 54—Identifying Patients and Patient Representatives in Community Pharmacy and Telepharmacy Settings (PDF, 178KB)
Section 9.6 – Protective Words
Section 9.2 – PharmaNet user enrolment and site registration in PRIME
General Policy Description
Under the Pharmaceutical Services Act and Information Management Regulation, all PharmaNet users must be granted access to PharmaNet by the Ministry of Health by enrolling in PRIME and all organizations must register their PharmaNet access sites in PRIME to be approved by the Ministry of Health to access PharmaNet.
If an individual is granted access to PharmaNet, it must only be used for the purpose of delivering health services in the user’s scope of practice (under the Health Professions Act or PODSA) directly to the individual whose record they access.
PRIME protects patient and health professional information.
Policy Details
Enrolling for individual access to PharmaNet
All health professionals who need access to PharmaNet must enrol in PRIME and renew their enrolment every year. Under the IM Regulation, if any information collected in PRIME changes (e.g., name, care setting, licence type), PharmaNet users must log in to PRIME and update their profile within 7 days of the change. Users who do not require access to PharmaNet for a period of 90 days or longer must record the absence in PRIME, and advise when they again require access.
In PRIME, health professionals sign the PharmaNet user terms and conditions assigned to them. There are different versions of the terms of access that apply to different types of PharmaNet users.
PRIME assigns a unique global PharmaNet ID code (GPID) to every health professional who is approved to access PharmaNet. Once approved for access, the GPID is shared with their workplace’s PharmaNet software vendor or administrator, who sets up their PharmaNet access. The GPID must be included in the local PharmaNet user profile, and is automatically included on every transaction they make in PharmaNet.
All individuals must be 18 years of age or older to access PRIME.
For guides and links to enrol in PRIME, visit:
Registering a site for PharmaNet access
Pharmacies, community health practices, device providers and health authorities must register every PharmaNet site in PRIME.
A PharmaNet site is a physical location where PharmaNet is used to deliver in-person patient care, plus a PharmaNet software vendor used at that location.
For guides and links to register a site in PRIME, visit:
- Pharmacy access to PharmaNet
- Community health practice access to PharmaNet
- Device provider access to PharmaNet
- Health authority facility access to PharmaNet
Community pharmacies are only permitted one connection to PharmaNet, using a single approved PharmaNet software application. If they use a different application to access other systems or information, such as laboratory results, any PharmaNet component of that application must be inactive.
Site registrations in PRIME must be kept up to date.
Remote access to PharmaNet
The option to access PharmaNet from outside a community health practice site (e.g., from home) is only available to physicians and nurse practitioners working in private community health practices, and as an addition to access at the practice.
Such access is only granted as an explicit exception under the IM Regulation, and both approved remote users and the organizations responsible for their associated PharmaNet sites must adhere to specific requirements relating to remote access.
Remote access must be linked to the care of patients of the practice.
Before remote access can occur:
- The private community health practice has to identify potential remote user(s) in the site’s PRIME registration
- The PharmaNet user must indicate that they require remote access in their PRIME user enrolment
- If a physician or nurse practitioner provides care at multiple private practices and wants remote access for all or several of them, each practice site must register them as a remote user, and the remote user must add each practice to their PRIME profile. It is the responsibility of the user to ensure they use the correct site’s connection when they work remotely
All remote access must be done using software approved by the Ministry of Health for this purpose.
On-behalf-of users are never eligible for remote access to PharmaNet, even if they access PharmaNet on behalf of a physician or nurse practitioner who is a remote user.
Remote access for health authority users
If a remote access exception is made for PharmaNet users in health authority facilities, scope and administration of remote access must be included in the Organization Agreement for Use of PharmaNet that is signed directly between the health authority and the Ministry.
Section 9.3 – Access to Patient Information in PharmaNet
General Policy Description
Access to patient information in PharmaNet is subject to the Pharmaceutical Services Act and the Information Management Regulation.
Policy Details
Access at community pharmacies
Under the Pharmaceutical Services Act and its Information Management Regulation, pharmacists and pharmacy technicians may access medical and claims history in PharmaNet if they are working at a pharmacy that is enrolled as a PharmaCare provider.
Individuals such as pharmacy assistants who are accessing PharmaNet on behalf of a pharmacist must do so at the direction of the pharmacist and under the same requirements and scope as the pharmacist they are acting on behalf of at the time.
Under the Pharmacy Operations and Drug Scheduling Act (PODSA) Bylaw 35(2) (PDF, 353KB), registrants of the College of Pharmacists of BC (CPBC) can only access a patient's PharmaNet record:
- To dispense a drug
- To provide patient consultation
- To evaluate a patient's drug usage, and/or
- For the purposes of claims adjudication and payment by an insurer
Access by patients
A patient may request a printed copy of their PharmaNet patient record (dispensing history) through a pharmacy or the Ministry of Health, or they can view it in Health Gateway. They can also request a copy of their record from a local pharmacy, which would contain only the medications that were dispensed by that pharmacy.
While a community pharmacy can print a patient’s locally stored record, it cannot print the PharmaNet patient record. Instead, the pharmacy submits the request to the Ministry of Health. Pharmacists can use their local system software to request the PharmaNet record.
If the client requests the record through the pharmacy, it gets mailed to them. If they request it through the Ministry of Health, they must go to a pharmacy to pick it up so the pharmacy can confirm the correct person receives the profile.
Patients may use HLTH 5551 - Consent for Release of PharmaNet Patient Record (PDF, 998KB) to request either:
- A current PharmaNet patient record, which includes the patient’s demographic information, clinical information, adverse drug reaction information, and medication history of all the dispenses within the last 14 months, or
- An archived PharmaNet patient record, which includes a record of medications dispensed beyond the past 14 months or in a specific date range. Records can be requested from September 1, 1995, onward (when PharmaNet was implemented)
- The PharmaNet patient record does not contain information about the cost of the medication or how much was covered by PharmaCare. For this information, clients can submit an HLTH 2942 - Authorization to Release Medical Records (PDF, 533 KB)
Each access to a patient profile in PharmaNet is recorded even when there is no associated dispense. A printed PharmaNet patient profile provided by the Ministry of Health or a pharmacy will include a list of all accesses made to the patient’s profile, including access that is not associated with a prescription fill.
Patients may also freely access their PharmaNet medication dispensing history as far back as 1995 online using their Health Gateway account. The Gateway does not include all fields of the patient’s PharmaNet record, or the record of accesses made to the profile.
Related resources:
HLTH 5551 - Consent for Release of PharmaNet Patient Record (PDF, 998KB)
How to get your PharmaNet patient record
Access for research
Access to PharmaNet patient information for health research is subject to the Pharmaceutical Services Act. All research access to PharmaNet, including clinical research, must be approved by the Ministry of Health’s Data Stewardship Committee (DSC). The DSC also reviews requests to use PharmaNet data to contact individuals to request participation in health research prior to their assessment by the Office of the Information and Privacy Commissioner.
Visit Apply for Data Access for further information.
PharmaNet access restrictions
These restrictions ensure patient and practitioner privacy are protected according to B.C. legislation.
Locale and site access restrictions
Unless a specific exception is made by the Ministry of Health, all access to PharmaNet must occur when the user is physically located at an approved PharmaNet site, and access by on-behalf-of users must occur when they are located at the same approved site as the registrant on whose behalf a given access is made.
Under the Information Management Regulation, all access to PharmaNet must occur when the authorized PharmaNet user is physically located in B.C. There are no exceptions.
Any online PharmaNet claim must be processed by the B.C. site (that is, it cannot be processed remotely from another location), and any personal information derived from that claim or from PharmaNet in general must remain in B.C.
Physicians and nurse practitioners working in private community health practices may request approval from the Ministry of Health to access PharmaNet outside of a registered site, if the registered site has first been approved to provide it to them. Refer to Remote access [linked to section above]
Device restrictions
All devices used to access PharmaNet must comply with privacy and security requirements set by the Ministry of Health. Any used for remote access must also comply with the specific requirements for remote access. Both are included in organization agreements and user terms of access.
Section 9.4 (Removed)
Section 9.5 – Patient Records
General Policy Description
Patient information is stored on PharmaNet, the province-wide network that links all B.C. pharmacies to a central data system since 1995. PharmaNet is administered by the Ministry of Health. Prescriptions dispensed in B.C. are entered into PharmaNet.
Policy Details
Patient data stored on PharmaNet
When a prescription is dispensed, the following patient information is recorded:
- Name and address
- Date of birth
- Personal Health Number (PHN)
- Adverse drug reactions
- Drug allergies
- Any clinical conditions the patient may have
- Details of all prescription medications, including veterinary medications dispensed in a community pharmacy to an individual’s pet(s)
Up to the last 14 months of patient medication history is available online from PharmaNet.
Patient record access
The details of patient’s prescriptions stored on PharmaNet are available to approved users granted access by the Ministry of Health for clinical purposes, unless a patient protective word has been assigned restricting access.
For more information on access to patient records, refer to Section 9.3—Access to Patient Information.
A patient may choose to restrict access to their medication history by assigning a protective word to limit access to their records.
For further information, refer to Section 9.6—Protective words.
Health regulatory bodies may have access to data from PharmaNet, in order to monitor and regulate their respective professions.
For more information, refer to Section 9.3—Access to Patient Information.
Patient requests to view their own data
A patient may request a printed copy of their confidential and personal data (either the data stored on the local system, the data stored on PharmaNet, or both). A patient may make the request through any community pharmacy. If the patient wants a PharmaNet record earlier than the last 14 months, or for a specific date range, they must contact the PharmaNet Profile Services Team directly.
The PharmaNet Profiles Services Team is available at:
Email: PharmaNetProfiles@gov.bc.ca
Phone toll-free: 1-855-952-1432
Fax: 250-953-0432
Mail: PO Box 9652 STN PROV GOVT
Victoria, BC, V8W9P4
Although the local system record may be printed at the community pharmacy, the PharmaNet patient record may not be printed.
Requests for PharmaNet data may be sent via PharmaNet to the Ministry of Health using the Patient Access to Personal Data function (TPM transaction). The Ministry of Health then mails the information directly to the patient.
The Ministry of Health requires pharmacists/medical practices to validate the patient’s address and PHN on PharmaNet (TID transaction) and update the address (TPA transaction), if necessary, before requesting a mailing.
Patients who want a printed record of PharmaNet data earlier than the last 14 months, or for a specific date range, must make the request directly to the PharmaNet Profile Services Team at 1-855-952-1432. The Services Team mails the profile to the patient’s pharmacy to verify the patient’s identity before its release. Both the pharmacist and patient must then sign a release form, to be faxed back to the Ministry of Health for confirmation.
The PharmaNet information sent to a patient includes:
- Patient demographic and clinical information
- Patient adverse drug reaction information
- Patient medication history for the specified date range, including veterinarian medications dispensed in community pharmacy (as far back as 1995)
- A log of all persons who have accessed the patient’s information, including when no medication was dispensed
The medication history normally includes only medications from community and hospital out-patient pharmacies.
>> Refer also to Section 9.3—Access to Patient Information.
Procedures for Pharmacists and Medical Practices
Any B.C. resident may request a printed copy of their confidential and personal data, whether it be for information stored on a pharmacy's local system or the data stored on PharmaNet.
The local patient record may be printed at the pharmacy.
Patients can request a copy of their current PharmaNet patient record (up to the last 14 months) through their community pharmacy at no charge.
To process a patient's request for their PharmaNet record from the last 14 months:
- Positively identify the patient.
>> Refer to Section 9.1—Positive Identification of Patients for more details - Validate the patient’s address and PHN on PharmaNet (TID transaction) and update the address (TPA transaction), if necessary, before requesting a mailing.
Validation of a patient’s identification and address is mandated by the College of Pharmacists of BC to protect patient privacy and ensure the profile is mailed to the correct address. - Send the request via PharmaNet using the Patient Access to Personal Data function (TPM).
Depending on your pharmacy software, this function may be labelled in the menu as “Request Profile Mailing.” Please contact your software vendor if you need assistance with the procedure.
The request is automatically forwarded to the Ministry of Health. The Ministry of Health will mail the information directly to the patient.
Patients can also request their record from earlier than 14 months, directly from the PharmaNet Profile Services Team.
To process a patient’s request for their PharmaNet record earlier than the last 14 months:
- Advise the patient to call the PharmaNet Profile Services Team directly at 1-855-952-1432. This is for data earlier than the last 14 months or a specific date range
- When the printed record arrives at the pharmacy, positively identify the patient.
Refer to Section 9.1—Positive Identification of Patients for more details - Both you and the patient must sign the release form, then fax it back to the Ministry of Health to confirm the records have been provided to the patient
For patients who would like to view their record online, they can access it through Health Gateway, providing they are a B.C. resident who has activated the BC Services Card app.
Section 9.5 Tools and Resources
- CPBC: PharmaNet Patient Record
Section 9.6 – Protective Words
General Policy Description
A patient has the option of attaching a protective word to his or her PharmaNet patient record. The protective word limits access to the patient’s record to only those pharmacists and authorized health practitioners to whom the patient provides the protective word.
Policy Details
Protective word requirements
The Pharmaceutical Services Act and its Information Management Regulation establish that at the request of an adult patient, a pharmacist must establish, delete or change the patient's PharmaNet protective word. Other requests (from minors or requests for protective words on another patient’s record) must be submitted in writing to Health Insurance BC.
Protective words are not required but all patients must be informed of the protective word option.
Patients must be informed of, and understand, the importance of keeping PharmaNet protective words confidential.
Patients must be made aware that they cannot apply a protective word to only selected parts of their record (e.g., they cannot prevent users from viewing only specific prescriptions in their medication history). The patient’s entire record is either restricted by the protective word or not.
Patients must also be informed that the protective word may be overridden by authorized health care practitioners in an emergency situation where they are unable to provide it. See Emergency Access if Protective Word Not Known.
Hospital Access to PharmaNet, Emergency Department Access to PharmaNet, and Medical Practice Access to PharmaNet require compliance with the applicable PharmaNet Professional and Software Conformance Standards with regard to patient protective words. Refer to the Conformance Standards for PharmaNet website for further details.
Access to protected PharmaNet records
After the protective word is set, it must be provided when
- Accessing a patient’s medication history
- Performing a Drug Use Evaluation (DUE)
- Dispensing a prescription
- Viewing any information about a patient’s prescriptions (e.g., number of refills remaining, allergies recorded, prescription costs, etc.)
- Submitting a request on a patient’s behalf to have their PharmaNet Medication History mailed to them (pharmacists only)
- Changing a protective word (pharmacists only)
A patient’s physician must provide the protective word when calling the pharmacist for information contained on a patient medication history.
The pharmacist may use the patient record on the pharmacy’s local system, when necessary, without a protective word.
Emergency access if protective word not known
Only an authorized regulatory body, such as the College of Pharmacists of BC, is able to enter a patient file without the protective word (for regulatory monitoring purposes only).
In an emergency, however, if a patient is unconscious or unable to provide their protective word, authorized health care practitioners can contact the PharmaCare Help Desk at Health Insurance BC to have it removed if they determine access to the patient’s PharmaNet patient profile is necessary for safe and effective treatment.
Within a reasonable period of time after the protective word has been removed, the patient will be notified that it has been removed and that they must request a new word be applied, if they so choose.
Who may request a protective word at a community pharmacy
Any person who is 19 or older can apply in person at a community pharmacy to update their PharmaNet record by adding, removing or changing a protective word.
>> See Procedures for Pharmacies below for details.
All other persons must apply in writing to Health Insurance BC. This includes the following:
- Minors (under 19) who wish to update their own PharmaNet record
- Persons (such as the guardian of a minor, or a person who is legally authorized to make decisions on another adult’s behalf) who wish to apply, remove or change a protective word on another person’s PharmaNet record
>> Refer these patients to the Protective word for a PharmaNet Record page on the PharmaCare website. It contains the necessary guidelines and application forms.
Acceptable proofs of identity when managing patient protective words
The following identification requirements apply when managing patient protective words (i.e., when applying, removing or changing a protective word on the patient’s own PharmaNet record).
Acceptable pieces of identification are set forth in the British Columbia Office of the Chief Information Officer’s Evidence of Identity Standard: Section 3 (PDF, 1420KB). The tables below summarize the acceptable pieces of identification.
All proofs of identification must be originals, not photocopies.
| Document Type | Requirements/Restrictions |
|---|---|
| Option 1 | |
| BC Services Card with the patient's photo | Must be valid (not expired) Must display a recent (within 5 years) photo |
| Option 2 | |
| BC Services Card without photo | Must be valid (not expired) |
| OR | |
| BC CareCard | |
| AND one of the following pieces of government-issued photo ID | |
| Canadian or U.S. driver's licence, learner's licence or enhanced driver's licence | Must be valid (not expired) Must display a recent (within 5 years) photo |
| BC Identification (BCID) card or enhanced identification card | Must be valid (not expired) Must display a recent (within 5 years) photo |
| Passport (Canadian or foreign) | Must be valid (not expired) |
| Foreign government passport | Must be valid (not expired) |
| U.S. passport card | Must be valid (not expired) |
| Canadian citizenship card | Must be valid (not expired) Must display a recent (within 5 years) photo |
| Canadian permanent resident card | Must be valid (not expired) Must display a recent (within 5 years) photo |
| Canadian Forces identification | Must be valid (not expired) Must display a recent (within 5 years) photo |
| Royal Canadian Mounted Police identification | Must be valid (not expired) Must display a recent (within 5 years) photo |
| Secure Certificate of Indian Status | Must be new secure version issued after 2009. Certificate of Indian Status cards issued prior to 2009 are not accepted Must display a recent (within 5 years) photo |
| Any other credential or evidence approved by the Chief Information Officer for the Province of British Columbia | Where an individual is ineligible for one of the required credentials, additional credentials or evidence may be accepted where approved by the Chief Information for the Province of British Columbia as providing equivalent assurance. |
When names do not match
When the name on the patient’s photo ID does not match the name in the PharmaNet record or on one or more other pieces of identification, the patient must provide additional documentation to establish a link between the two names.
Acceptable Proof of Identity: Change of Name
| Document Type | Requirements/Restrictions |
|---|---|
| Change of name certificate | Must be issued by Canadian province or territory’s registrar of vital statistics |
| Marriage certificate, certified statement of marriage, or record of marriage form | Must be issued by Canadian Province or Territory’s Registrar of Vital Statistics; or Clergy member, judge or justice of the peace that performed the marriage Must be signed by the person who performed the marriage Must contain the name of both spouses, the date of the marriage and licence number |
| Any other credential or evidence approved by the Chief Information Officer for the Province of British Columbia | Where an individual is ineligible for one of the required credentials, additional credentials or evidence may be accepted where approved by the Chief Information for the Province of British Columbia as providing equivalent assurance. |
Protective word removal
If an adult at a pharmacy has forgotten their protective word, a pharmacist can ask the PharmaCare Help Desk to remove it.
If the patient’s protective word is removed, Health Insurance BC will notify the patient in writing.
The protective word can be changed only once in 24 hours but—should a patient lose or forget the protective word—the PharmaCare Help Desk may remove it.
Creating a protective word
A patient must choose their own protective word which is then entered on PharmaNet by the pharmacist. A pharmacist cannot choose a protective word for a patient.
The pharmacy must have policies and procedures in place to protect patient privacy when providing protective words (e.g., if the protective word cannot be spoken in a confidential manner, the pharmacy may provide pen and paper, disposing of the paper appropriately after use).
The patient’s protective word must contain the following:
- Six to eight characters (no spaces allowed)
- Letters and numbers only (no special characters such as #,\ and &)
- At least two letters (A…Z)
- At least two numbers (0…9)
Examples: PA6729BC, 90PAMA17, FOTO2609
Tips: Patients should create a word that is easy for them to remember but hard for someone else to guess. They should NOT use their mother’s maiden name, their birth date or their phone number. There is an increased likelihood of the information being discovered by an outside source and used to access PharmaNet information.
Patients should be encouraged to write down their protective word and store it in a safe place.
Storing and sharing patient protective words
Protective words can be stored in the local system only with prior, explicit, consent from the patient. The individual should be advised that all pharmacy staff will be able to view and use the protective word and will not need to ask the individual for it before accessing their PharmaNet record.
Protective words can be stored only in encrypted form in the local system’s software. Consult your software documentation for directions.
Protective words must not be stored on paper. If a patient writes out the protective word for you to enter into the system, you must either (a) return the paper to the patient for destruction or (b) immediately destroy it in a secure fashion.
Protective words must not be shared with any other person or facility. They must not be made available to any other user across a shared network (e.g., pharmacy chain).
You must remove a stored protective word from the local system immediately if the patient requests it.
Procedures for Pharmacies
Adding a protective word to a patient's own PharmaNet record
The patient must be 19 or older and must provide adequate proofs of their identity before you can process their request.
- Review the patient’s proofs of identity and confirm their age. If you have any concerns about these proofs, refer the patient to Health Insurance BC.
- Provide a private location in which the patient can provide you with a protective word that meets the rules above or have them write out the protective word so that it cannot be overheard.
- Attach the protective word to the patient’s PharmaNet record using the Patient Keyword Maintenance (TCP) transaction.
- If applicable, destroy the paper bearing the protective word.
- Inform the patient that the change takes effect immediately.
Removing a protective word from a patient's own PharmaNet record
The patient must be 19 or older and must provide adequate proofs of their identity before you can process their request.
- Review the patient’s proofs of identity and confirm their age. If you have any concerns about these proofs, refer the patient to HIBC.
- Telephone the PharmaCare Help Desk to remove the protective word.
- Inform the patient that the change takes effect immediately.
Changing a protective word on a patient's own PharmaNet record
A patient can apply to change a protective word only once in a 24-hour period.
The patient must be 19 or older and must provide adequate proofs of their identity before you can process their request.
The patient must provide you with their current protective word for you to process the change using the Patient Keyword Maintenance (TCP) transaction.
If they cannot remember the word, telephone the PharmaCare Help Desk to have them remove the current protective word. Once it is removed, you can use the Patient Keyword Maintenance (TCP) transaction to add a new protective word.
When the patient can provide their current protective word
- Review the patient’s proofs of identity and confirm their age. If you have any concerns about these proofs, refer the patient to HIBC.
- Provide a private location in which the patient can provide you with a protective word that meets the rules above or have them write out the protective word so that it cannot be overheard.
- Attach the protective word to the patient’s PharmaNet record using the Patient Keyword Maintenance (TCP) transaction.
- If applicable, destroy the paper bearing the protective word.
- Inform the patient that the change takes effect immediately.
When the patient cannot provide their current protective word
- Review the patient’s proofs of identity and confirm their age. If you have any concerns about these proofs, refer the patient to HIBC.
- Telephone the PharmaCare Help Desk at HIBC to remove the patient’s current protective word. Cite the type of identification provided. The change will take effect immediately.
- Provide a private location in which the patient can provide you with a new protective word that meets the rules above or have them write out the protective word so that it cannot be overheard.
- Attach the protective word to the patient’s PharmaNet record using the Patient Keyword Maintenance (TCP) transaction.
- If applicable, destroy the paper bearing the protective word.
- Inform the patient that the new protective word takes effect immediately.
Section 9.6 Tools and Resources
Section 9.7 – PharmaNet Security
General Policy Description
PharmaNet has many built-in security features to prevent unauthorized access to patient information—including data encryption, a “firewall” to prevent outside access to restricted files, and a system of tightly monitored access privileges.
PharmaNet was developed to address the diverse needs of individuals and organizations using it. Some users need “update” access to specific parts; others may require “read-only” access to specific information.
Policy Details
PharmaNet has several levels or layers of access security. Each user and organization has been granted the level of access required to perform their role in managing and using the system.
Access to PharmaNet requires adherence to security requirements of the applicable PharmaNet Professional and Software Conformance Standards. Refer to the Conformance Standards website for further details.
The levels of security are as follows:
- Physical security
- Operating system security
- PharmaNet security
- Transaction security
- Screen security
Physical security
Physical security addresses physical access to the hardware components of PharmaNet. Physical security includes:
- Restricted access to premises in which the hardware resides
- Logging of all access to the PharmaNet equipment
- Inventory checking
- Security procedures for handling and storage of backup and storage media (tapes, discs, flash and hard drives, etc.)
Operating system security
Operating-system security regulates the security of the operating system, for example, by controlling user access and associated privileges, and by allocating PharmaNet system resources to allow PharmaNet to meet performance levels.
Included in operating-system security are:
- The assignment of required user IDs
- The establishment and administration of user password standards and policies
- The assignment of system resources to users with appropriate security clearance
PharmaNet security
PharmaNet API security protocols prevent access to PharmaNet by unauthorized users and unauthorized interception of data traveling to and from the PharmaNet system.
PharmaNet’s core system is behind a firewall on the provincial government’s servers. All PharmaNet access sites are connected through the PharmaNet API.
Information or messages being transmitted are monitored and authenticated to ensure they are from authorized users. In addition, all personal information that may identify an individual is encrypted to prevent monitoring of the transaction data.
Transaction security
Transaction security grants or limits services to authorized individuals or groups. Each organization connected to PharmaNet is assigned a group of privileges based on the type of PharmaNet transactions it will use. This group of transactions represents the precise services that a specific organization is eligible to use.
PharmaNet screen security
Screen security controls internal user or group access to specified PharmaNet database screens and the ability to perform specific functions on those screens.
This description applies to internal screens that are part of PharmaNet, not the vendor-supplied screens that are located externally at pharmacies.
Based on each internal user’s security profile, certain menu items, functions, screens, etc., may be made inaccessible. Some groups may be allowed to change or add information, other groups may only be able to read or view the information and others may have no access at all.

10 - Audit
Section 10.1 – Audit Policies
General Policy Description
Audits are performed to ensure providers—and claims for drugs, medical supplies, and services paid by PharmaCare to a provider—are in compliance with the terms of relevant Acts, regulations, bylaws, policies and procedures.
Policy Details
Application of policy
This policy applies to all providers enrolled in PharmaCare by way of:
- PharmaCare Enrolment Agreement
- Pharmacy Participation Agreement
- British Columbia PharmaCare Non-Pharmaceutical Supplier Participation Agreement
- British Columbia PharmaCare Pharmacy Participation Agreement for the Provision of PharmaCare Services to Long Term Care Facilities
- Methadone Maintenance Payment Program Addendum to Pharmacy Participation Agreement, and
- Pharmaceutical Services Act and regulations.
Issues subject to audit
The Pharmaceutical Services Act and Provider Regulation establishes the following with regard to audit:
- The Minister may appoint inspectors to conduct audits and inspections for the following purposes:
- to determine compliance with the Pharmaceutical Services Act
- to fulfill a prescribed purpose.
- The following matters may be the subject of an audit or inspection:
- a claim
- the billing and business practices of a person referred to above
- prescribed matters
An inspector may audit or inspect in respect of:
- A provider, a manufacturer, a supplier, a franchisor or an alternate payee
- A former provider, manufacturer, supplier, franchisor or alternate payee
- A person who is prohibited from providing or receiving incentives under the Act
- A person who was formerly a person who is prohibited from providing or receiving incentives under the Act.
Claims subject to audit
All PharmaCare claims are subject to audit to confirm compliance with the provisions of the following:
- The Pharmaceutical Service Act;
- The Health Professions Act;
- The Pharmacy Operations and Drug Scheduling Act;
- A prescribed enactment of British Columbia or Canada; or
- The regulations made, or a limiting condition imposed, under any of the above Acts
Note: The above list includes College of Pharmacists of BC bylaws, PharmaCare policies and procedures, and policy and procedural updates communicated in PharmaCare Newsletters which the above Acts, enactments, regulations or limiting conditions require providers to follow.
Audit inspectors
Audits are performed by PharmaCare Audit and Audit Intelligence and Operations, Audit and Investigations Branch.
PharmaCare auditors are appointed as inspectors by the Minister of Health pursuant to the Pharmaceutical Services Act for the purposes of conducting audits.
Audit access
Under the Pharmaceutical Services Act, a person who is subject to an audit must do all of the following on the request of the audit team:
- Produce or provide electronic access to, and permit inspection of, the records requested by the audit team;
- Supply copies of or extracts from the records; and
- Answer all questions of the audit team respecting any matter relating to the records or to the audit generally.
If records that are required are not located on the premises, the person who has possession of those records must produce and permit inspection of those records if requested by the audit team.
If the audit team is not provided with sufficient information to conduct the audit, an adverse inference may be drawn. The result may be a denial of claims for which the audit team has not been provided sufficient information.
In addition, knowingly providing false or misleading information to the audit team, or willfully interfering or obstructing the audit team constitutes an offence under the PSA. A person who commits such an offence is liable on conviction to a fine up to $200,000 or to imprisonment for a term up to six months, or to both.
PharmaCare auditors will complete a Temporary Removal of Documents form with the manager of the provider site being audited when records are removed.
In the event that PharmaCare auditors remove any records from a provider site or the location where the records are kept, the records will be returned within 20 business days.
Access to electronic records for audits and inspections
For electronic records to be deemed accessible, inspectors must be able to sort and filter the records based on one or more of the following data elements:
- PHN
- Date/time of image creation
- Date/time of service
- Prescriber ID
- Original prescription number
- Transaction number
- Record type (e.g., Frequent Dispensing Authorization forms)
The point of sale (POS) system must support export of a record or collection of records (a set of images) usable for subsequent reference. The collection of records must be able to be transferred off-site via secure file transfer processes or via encrypted email for small files. The transfer of encrypted emails must be confirmed before the data transfer. Both transfer methods must be compliant with all relevant legislation.
The export images must be PDF. The file name must include the following, separated by dashes (-):
- PHN
- Prescription number, and
- Date/time of image creation
Provider recordkeeping requirements
Providers must abide by the record-keeping requirements as specified in the Pharmaceutical Services Act and Regulations, including Section 12, 13 and 14 of the Provider Regulation.
For the purposes of calculating non-compliant claims amounts, information a provider obtains from a prescriber/other provider after an onsite audit cannot be used to support a disallowed prescription claim.
Electronic recordkeeping
The PharmaCare program allows electronic recordkeeping if the provider meets the standards set out in this policy.
In scope:
- Community pharmacies, including those that provide general medical supplies, and pharmacies enrolled in the device class (see section 5.2 of the PharmaCare Provider Enrolment Guide)
- Paper prescriptions supporting PharmaNet records
- PharmaCare forms and College of Pharmacists of British Columbia (CPBC) forms listed at the end of this section, and any other forms that support PharmaCare claims
- PharmaCare claims records that pharmacies have transferred to another pharmacy and the records of pharmacies that have closed
Out of scope:
- If the limits or conditions on a pharmacy’s enrolment as a PharmaCare provider require it to maintain hardcopy original records, then it may not keep electronic records only
- Non-pharmacy device providers submitting claims. The PharmaCare Prosthetics and Orthotics Policy Manual requires the retention and provision of original hardcopy documentation
- Prescriptions for drugs in the controlled prescription program and part-fill accountability logs associated with opioid agonist treatment prescriptions
- Hospital pharmacies with outpatient services, not licensed as community pharmacies
- Electronic prescribing, defined as the secure electronic creation and transmission of a prescription between an authorized prescriber and a patient’s pharmacy of choice, using clinical electronic medical record (EMR) and pharmacy management software
Policy:
Electronic recordkeeping does not diminish the responsibility to maintain records or comply with obligations under the Pharmaceutical Services Act (PSA). The following standards apply to all electronic records supporting PharmaCare claims, including prescriptions, PharmaCare forms and certain CPBC forms (listed at the end of this section). Any claims based on digital-only records that do not meet these standards will be subject to full recovery.
Please note that in this policy, the terms "electronic record” and “digital record” are used interchangeably since both terms are used in related documents (Pharmacy Operations and Drug Scheduling Act (PODSA); Health Professions Act (HPA); and PharmaNet conformance standards).
Where applicable, PharmaCare’s requirements are supported by technical requirements defined in the Ministry of Health Conformance Standards, Volume 4C–Application Enforced Rules for PharmaNet. This volume of the conformance standards defines the application rules that must be enforced by POS systems when accessing PharmaNet.
New rules were added on September 30, 2021 to Volume 4C, section 3.6.1 (version 3.3) of the conformance standards to support electronic recordkeeping, although as of the writing of this policy the conformance testing of these rules had not been completed and a deadline for conformance testing had not been established. Further, the conformance standards and associated rules are subject to change.
Provision and retention of original records
The PharmaCare program’s requirement for providers to retain original records in digital form is satisfied if there exists reliable assurance as to the integrity, legibility and accessibility of the record.
Records must be maintained for the longest applicable period stipulated by the PODSA bylaws or the Provider Regulation (under the PSA) at a minimum. In effect, this is 4 years for 1-year prescriptions, 5 years for 2-year prescriptions (i.e., birth control), and 4 years for PharmaCare forms, or until an audit or inspection is complete, whichever is longer.
If storing digital records for paper/fax prescriptions and/or PharmaCare record types in the point of service (POS) system, each of the requirements described in the PharmaNet conformance standards (3.6.1 PNetTx8.2 and PNetTx9.1) must be met. Should these requirements be amended in the future, the amended requirements must be met.
- PNetTx8.2 Storing the Local Dispense Record
- PNetTx9.1 Digital Records for Paper/Fax Prescriptions and/or PharmaCare Record Types
Custody and control of information in digital records
When a pharmacy collects personal information and records it on a PharmaCare form, the pharmacy is creating its own record, over which it has custody and control. However, once the data in the record is submitted to the Ministry of Health (the Ministry), the Ministry will have custody and control of the submitted record. The pharmacy still has custody and control of any record remaining on its local system.
Record preservation and back-up
Digital records must be preserved and backed up at least once daily and stored in a location resistant to environmental perils including, but not limited to, fires and floods. They must be secure from unauthorized access, use, modification, destruction, and disclosure. The backed-up records, once restored, must also be compliant with applicable legislation, regulations, policies and bylaws.
Amendments to records
Once an electronic version of a record is created, the original electronic record must remain verifiably complete and unaltered and its integrity must be maintained. In the event a record is amended to correct an error or omission, the new record must be created as a separate document, with the original document and full evidentiary trail of changes maintained and accessible.
Recordkeeping policy and confidentiality undertakings
Documentation regarding the filing system adopted by the provider must be maintained by the provider and must be made available to the Ministry upon request. The documentation must include a description of the process for preservation, storage and back-up of electronic records, how they can be accessed by authorized individuals, and the effective date the provider adopted electronic recordkeeping.
The electronic records collected, used and retained on the pharmacy’s local POS system, contain personal and other information that can be easily disclosed when in an electronic format. The registrant must maintain confidentiality by disclosing the electronic record only for the purposes listed in the HPA Bylaws, s. 72. The CPBC requires the pharmacy manager, the registrant, the pharmacy support staff and the vendor to sign confidentiality undertakings to protect the confidentiality of both PharmaNet and pharmacy POS system records, including prescription and non-prescription records. In addition, the legislation, including the PSA, and bylaws are very clear that there may be no disclosure for market research.
Change in recordkeeping systems
In the event of a change in the recordkeeping system, the provider maintains responsibility for retention of and access to all records prior to the change. Any claims made based on records that cannot be produced will be subject to full recovery.
Transfer of records between providers
In the event of a change in provider ownership, the new owner is responsible for the retention of and access to the transferred records and the integrity of the records received from the previous owner.
Any claims based on invalid records or records that cannot be produced will be subject to full recovery.
Pharmacy closures
The policy remains in effect in the event of a pharmacy closure. If a pharmacy closes permanently, it is required to either transfer its records to another pharmacy or to a secure storage site. The owner of the closing pharmacy remains responsible for all records in storage for their retention time as required by applicable legislation, regulations and bylaws. The owner of the closing pharmacy is also required to produce records to support claims and is liable for full recovery. Transferred records become the responsibility of the owner(s) of the receiving pharmacy.
Any claims made based on invalid records or records that cannot be produced will be subject to full recovery.
Definition of signature
For prescriptions, the requirements for signatures are defined by the CPBC.
For PharmaCare and CPBC forms that support PharmaCare claims and require prescriber, provider or patient signatures, there must be safeguards against repudiation, and the signature and process for obtaining the signature must definitively verify the identity of the signing party. The signature must be unique and applied by a human hand and must be an integral part of the original content. Records containing a stock electronic signature that is not unique or is not under control at all times of the individual whose signature it is are not acceptable. An image of a signature subsequently attached to the document is also not acceptable. Records with invalid signatures are subject to full recovery.
When paper originals are scanned for electronic storage, the “wet” signature of the prescriber, provider or patient is scanned as part of the document scanning process. This is a valid representation of the signature.
Image quality
Records stored electronically must include an image file that accurately reflects the prescription, form or other document, including the colour composition of that document; therefore, any physical records that are converted to an electronic record must be scanned in colour. The requirement for colour scanning ensures image quality requirements are met. If the record is not scanned as a colour image, it is not considered a valid record for audit purposes and will be subject to full recovery. The PharmaCare program’s colour requirement matches that of the HPA Bylaws s. 65.1(5) and PODSA Bylaws s. 23.1(5) and extends to any record supporting PharmaCare claims.
Readability
All necessary details available on a hardcopy document must be available and clearly legible on the electronic version of the record. Determination that a record does not meet the standard of readability will be made by the Ministry, in its sole discretion, at the point of review. If any elements of the record that are required for an audit or inspection are determined to be unreadable, the record will not be valid and any claim associated with the record will be subject to full recovery.
Access to electronic records for audits and inspections
Per ss. 36, 38 and 39 of the PSA, electronic records must be accessible by inspectors upon request.
For a record to be deemed accessible, the application must be able to search and export digital records as described in the PharmaNet conformance standards (3.6.1, PNetTx9.2 and PNetTx9.4). If the POS system allows users to sort and/or filter digital records of paper/fax prescriptions and/or PharmaCare record types, the application must meet PharmaNet conformance standards PNetTx9.3. Should these requirements be amended in the future, the amended requirements must be met.
- PNetTx9.2 Searching Digital Records
- PNetTx9.3 Sorting and/or Filtering Digital Records
- PNetTx9.4 Exporting Digital Records
Failure to produce electronic records
If a claim is based on electronic records only and those records cannot be produced, the claim will be subject to full recovery.
Policy implementation:
The Ministry cannot require a pharmacy’s POS system to have the functionality outlined in the standards before conformance testing is completed. The standards were released on September 30, 2021, but the conformance testing deadline is not yet established.
However, some vendors have developed some related capacities in response to the College’s 2018 changes to its bylaws under the HPA and PODSA permitting an electronic-only option for storage of prescriptions and other records. If that functionality can be used to manage digital records before the conformance testing, and the pharmacy can meet the other requirements of this policy, the pharmacy is not required to keep hardcopy records.
If the system cannot meet the requirements of this policy, a paper copy of the records in scope for this policy must be retained and available for the purposes of inspection or audit.
Forward-facing application of policy:
This policy and its stipulations for electronic recordkeeping will only apply to pharmacy records from the policy’s implementation date (March 1, 2022). Records entered prior to implementation must meet previous standards; the new policy will not apply retrospectively.
List of records subject to the electronic recordkeeping policy
Note: Inspectors may review any form related to a PharmaCare claim; this list is only a sample.
- Original prescriptions (except controlled prescription program prescriptions)
- PharmaCare forms
- Travel Declaration (acquired through HIBC)
- HLTH 5425 - Compound Costing Worksheet (PDF, 521KB)
- HLTH 4571 - Plan W OTC Recommendation (PDF, 932KB)
- HLTH 5336 - PharmaCare Claim
- HLTH 5335 - PharmaCare Prescription Invoice
- College of Pharmacist of BC (CPBC) Forms
- Pharmacist Prescription Adaptation Documentation and Notification Form (PDF, 82KB) or equivalent documentation
Note that the CPBC OAT Part-Fill Accountability Log is not included in the electronic recordkeeping policy at this time, as the hardcopy records of a prescription for drugs included in the controlled prescription program are also excluded from electronic recordkeeping.
Recovery of non-entitled amounts
The Pharmaceutical Services Act establishes the following with regard to recoveries:
- An amount is a non-entitled amount if the amount is paid by the Minister to a provider or an alternate payee, or a former provider or alternate payee, who, under the Pharmaceutical Services Act, is not entitled to the amount, including any amount paid
- for a drug, device, substance or related service provided to a person who was not a beneficiary, at the time of the claim
- for a drug, device, substance or related service that was not a benefit,
- in respect of a claim for payment
- for a benefit that was not provided, or
- that is not supported by the records kept or produced under this Act,
- after relying on a representation of fact that was untrue,
- by mistake, or
- if, in providing the benefit or making the claim, the person acts contrary to
- the Pharmaceutical Service Act
- the Health Professions Act
- the Pharmacy Operations and Drug Scheduling Act
- a prescribed enactment of British Columbia or Canada;
- the regulations made, or a limiting condition imposed, under any of the above Acts.
Note: The above list includes the College of Pharmacists of BC bylaws, PharmaCare policies and procedures, and policy and procedural updates communicated in PharmaCare Newsletters, which the above Acts, enactments, regulations or limiting conditions require providers to follow.
- Without limiting any action the minister could take under section 46 [enforcement orders] of the Pharmaceutical Services Act, if the minister determines that a non-entitled amount was paid to a provider, the minister may require the provider to
- repay the non-entitled amount,
- pay a prescribed surcharge, and
- pay interest on the amounts owing due to non-entitled amounts or prescribed surcharges.
- The total amount that a person is liable to pay under the section above is a debt due to the government and may be
- deducted from any subsequent payment that may be made to the person under this Act, including under an agreement made under this Act, or
- recovered in a court of competent jurisdiction.
- Where any amount is found to be owing by the Provider to the Province, the Province may require and the Provider shall repay, no later than thirty (30) days from the receipt of the demand, the amount owing.
- Without limiting other remedies available to the Province at law, if the Provider fails to make any repayment required under the section above, the amount owing may be deducted from any money owing by the Province to the Provider. The Province will collect the recovery by set-off (i.e. by deducting the recovery amount from a current or future payment) 30 days from the receipt of the amount owing.
Once a recovery amount is 30 days overdue it becomes subject to interest pursuant to the Financial Administration Act, Section 20: Interest on Overdue Accounts.
Selection for audit
Selection of a provider for audit may be made by statistical analysis and comparison of claims data, random selection, direct selection, or other means.
The method of selection will be identified in the Audit Reports.
Audit notification
Providers are informed by formal notice of an audit, including a letter—by fax, hand delivery or courier—confirming the auditors as duly authorized inspectors pursuant to the Pharmaceutical Services Act.
Audit sampling
An inspector when conducting an audit, may determine the results of an audit under the Pharmaceutical Services Act, in accordance with Ministerial Order number M066 dated March 11, 2015, including:
- Utilizing “Probability Proportionate to Size Sampling” (also known as “Monetary Unit Sampling” or “Dollar Unit Sampling”) to select samples of claims for audit testing.
- Selecting samples of claims using recognized statistical sampling software and/or related methodologies to select the sample on a systematic basis where the probability of selection is proportional to the size of the claim.
- Where the inspector determines it to be appropriate, stratifying the Population into sub-populations (known as "strata") where 100 percent of the claims in the stratum, or a sample of claims in the stratum, may be selected and examined.
- Selecting samples of claims with the objective of generating an estimate of the overpayment amount with a 90 percent confidence level.
- Preparing an estimate of overpayments in the population by calculating the average exception rate of all claims in the sample and extrapolating by applying the exception rate to the dollar value of the pharmacy's claims population. If the population is examined in two or more strata, separate exception rates are calculated from each stratum's sample and applied to each stratum's sub-population.
Draft audit report
The PharmaCare Audit inspectors prepare a Draft Audit Report for all audits they perform.
The Draft Audit Report identifies the:
- Preliminary results of the audit and the methodologies used to determine the results.
- Total of the claim amounts submitted by the pharmacy during the Audit Period for claims that were not in compliance with the Pharmaceutical Services Act and the methodology used to calculate this amount.
Providers have 60 days to respond to Draft Audit Reports by providing any further records, information or documentation that can confirm the audited claims were in compliance with the Pharmaceutical Services Act.
Provider responses to Draft Audit Reports are reviewed by the PharmaCare Audit inspectors and are considered prior to preparing the Audit Report.
Audit report
The results of the audit set out in the Audit Report are provided to the person subject to the Audit.
In the event of a recovery of non-entitled amounts, the covering letter of the Audit Report outlines the repayment options.
Repayment is pursued in the manner established in the Pharmaceutical Services Act.
Results of audits may be referred to the College of Pharmacists of BC or other regulatory bodies, if appropriate.
Confirmation Letter Program
The Confirmation Letter Program is used to randomly or selectively confirm PharmaCare claims information with patients or physicians.
Random confirmation letters
PharmaCare Audit mails a confirmation letter to a random sample of patients selected from a random sample of pharmacies.
The letter requests confirmation that the patient has received the medications or services that PharmaCare claims data identifies as having been dispensed to the patient in the previous months (refer to the sample confirmation letter below).
Results from returned confirmation letters are compiled and anomalies reported by patients (e.g., medications a patient indicates were not received) are investigated.
Select confirmation letters
Select confirmation letters may be used to support provider audits at the discretion of PharmaCare Audit.
Letters may be mailed to physicians or patients to verify PharmaCare claims information.
Results from returned confirmation letters are compiled and included in the audit file.
Section 10.1 Tools and Resources

11 - Contacts for Practitioners & Providers
11.1 - PharmaCare Help Desk and Medical Practitioner Line
The PharmaCare Help Desk provides support for pharmacists.
The Medical Practitioner Line provides support for prescribers.
Both services record, monitor, and resolve problems in a timely manner. Help Desk representatives do not answer questions about specific medical conditions or treatment.
Each call is assigned a ticket number, which you will need for any follow-up.
These numbers are for providers only. They should not be given out to the public.
Print off this poster for easy reference to the information in this section:
PharmaCare Help Desk
When you call the Help Desk, you will enter your College of Pharmacists of BC practitioner ID number (not employee number), then either use the self-service option or hold to speak to a representative, depending on the information you need.
Hours: 24/7 (closed only on December 25)
Phone: Lower Mainland: 604-682-7120
Rest of B.C.: 1-800-554-0225 (toll-free)
Fax: 250-405-3587
Mail:
PharmaCare Help Desk
PO Box 9655 Stn Prov Govt;
Victoria BC V8W 9P2
Information you need when calling
If calling about a practitioner, have on hand:
If you are calling about a specific client, you will need:
- Client’s full name
- Client’s PHN
Be prepared to provide:
- Pharmacy site ID
- Ticket number if issue was reported previously
Automated and in-person support depending on issue
Use the self-service options if you are looking for:
- Practitioner ID number. Press 1 and enter prescriber’s MSP billing #
- Special Authority details (e.g., max days’ supply, expiry date if applicable). Press 2 and enter client’s PHN
- Which plans cover a particular drug. Press 3 and enter drug DIN or PIN
- Client’s blood glucose test training status. Press 4 and enter client PHN
- Client’s coverage plans. Press 5 and enter client PHN
- Name of pharmacy that previously dispensed a prescription. Press 6 and enter client PHN
Hold to talk to representative to:
- Report a timeout or technical problem connecting to PharmaNet. Press 1
- Get information about a rejected claim and/or claims adjudication. Press 2
- Verify a patient’s MSP coverage or information on a BC Services Card. Press 3
- Get info about patient restrictions. Press 4
- Ask about a Minor Ailments and Contraception Service (MACS). Press 5
- Press 6 to speak with a representative about:
- Enrolment and PharmaNet setup for new providers
- Provider enrolment changes
- LCA shortages
- Electronic funds transfers (EFT)—new applications and changes
- Plan B capitation rates and payments
- Methadone interaction fees
- Pharmacy software vendor (PSV) changes
- IP address changes (including expanded ranges for new workstations)
Medical Practitioner Line
The Medical Practitioner Line supports prescribers with information about a client’s Special Authority, Plan G (Psychiatric Medications), Plan P (Palliative Care) coverage, and with the restricted claimant program.
Toll-Free: 1-866-905-4912
- Press 1: For Plan G Psychiatric Medications plan coverage or questions about Special Authority coverage, including coverage under the Reference Drug Program
- Press 2: For questions about Plan P Palliative care drug plan coverage
- Press 3: For the restricted claimant program
Problem assessment and escalation
The PharmaCare Help Desk assigns a severity level to each call, based on the problem’s impact on the pharmacy’s ability to conduct normal business. “Normal business functions” are defined as:
- Recording the dispensing of drugs
- Maintaining patient medication information
- Receiving DUE results
- Receiving PharmaNet adjudication results
A malfunction of a pharmacy’s local software or the PharmaNet network may interfere with one or more of these functions.
The four levels are:
Severity 1: A total inability to perform one or more normal business functions. All involved parties are expected to work continuously until the problem is resolved or the severity level is reduced.
Severity 2: All normal business functions work to some degree, but one or more are severely degraded. Work by all involved parties is expected to continue on a priority basis until a solution is in place.
Severity 3: All critical functions work, but one or more normal business functions are somewhat impaired.
Severity 4: The problem has been circumvented and is not seriously affecting normal business functions.
Escalation procedures depend on the severity level.
- High-severity problems that affect a single store are escalated to the PharmaCare Help Desk or Service Desk Supervisors
- Problems that affect a number of stores or the province as a whole are escalated to the Manager of Operational Support and from there to the Director of Service Experience.
HIBC informs the directors of PharmaNet Operations and Systems , IHSPS and Director of Operations, HPO whenever a pharmacy is unable to perform normal business functions.
The Service Level Agreement signed by each pharmacy software vendor defines escalation procedures and reporting structure between a pharmacy software vendor and the PharmaCare Help Desk. Not all calls are subject to the Service Level Agreement.
Appendix
Appendix A – Response Codes
- Appendix A Response Codes—PharmaNet adjudication response codes
Appendix B – Intervention Codes
- Appendix B Intervention Codes—PharmaNet intervention and exception codes
Appendix C – Reference Codes
- Appendix C Reference Codes—Practitioner ID reference codes for Canadian prescribers


