Oral Anticoagulants: Elective Interruption & Emergency Reversal
Effective Date: January 18, 2023
Recommendations and Topics
- Scope
- Key Recommendations
- Therapeutic Considerations
- Procedural Bleeding Risk
- Periprocedural Management for Warfarin
- Periprocedural Management for DOACs
- Rapid Reversal for Serious Bleeding or Urgent Procedures
- Therapeutic Agents for Rapid Warfarin Reversal
- Resources
- Appendices
Scope
This guideline provides recommendations for the management of direct acting oral anticoagulants (DOACs) and warfarin in patients aged ≥ 19 years who require an elective or urgent procedure or are actively bleeding and require emergency reversal.
Key Recommendations
- Whenever possible, procedures in an anticoagulated patient should be undertaken on an elective basis to allow for planned periprocedural anticoagulation.
- Whenever possible, discuss appropriate periprocedural anticoagulant management with the practitioner performing the procedure.
- Temporary interruption of an oral anticoagulant is not indicated for procedures with minimal risk of bleeding. All other procedures require temporary drug interruption. The duration of interruption is based on the type of anticoagulant, the risk of thrombosis, and the risk of bleeding related to the procedure.
- Bridging with low molecular weight heparin (LMWH) is only indicated for patients on warfarin with very specific and high- risk factors for thrombosis. Bridging is not indicated for DOACs.
- Rapid reversal of anticoagulants is indicated for patients with potentially life-threatening bleeding or patients who require an urgent/emergent procedure.
- Rapid reversal of warfarin is achieved with prothrombin complex concentrate (PCC) co-administered with vitamin K.
- Rapid reversal of dabigatran is achieved with idarucizumab.
- A specific agent for reversal of direct factor Xa (FXa) inhibitors (apixaban, edoxaban, and rivaroxaban) is not available in Canada. PCC has been used off-label for rapid reversal of these agents in life-threatening bleeding based on very weak evidence.
- In patients on chronic therapeutic dosing of a DOAC who undergo major surgeries that use prophylactic dosing of the DOAC post-operatively, timing of when to restart the therapeutic dose should be discussed with the surgeon.
 TOP
TOP
Therapeutic Considerations
Periprocedural management of oral anticoagulant (OAC) therapy requires balancing the risk of hemorrhage if the procedure is performed while on anticoagulation and the risk of thrombosis if anticoagulation is interrupted. Risk of hemorrhage is influenced by patient age, relevant medical conditions, type and site of procedure, approach, type of incision and closure, operator skill, and post-operative anesthesia (e.g., epidural). The risk of thrombosis depends on pre-existing conditions, time since the last episode of thrombosis, and the thrombotic effect of the procedure. There are no validated risk scores to accurately predict or measure the net outcome. Periprocedural anticoagulation recommendations are largely based on protocols evaluated in clinical trials, clinical experience, and expert consensus.1
Whenever possible, procedures in an anticoagulated patient should be undertaken on an elective basis to allow for planned anticoagulant reversal. Consider delaying elective procedures for patients with a recent thromboembolic event (e.g., within previous three months) to reduce the risk of recurrent stroke or thrombosis.
Rapid reversal of an OAC is necessary for patients presenting with potentially life-threatening bleeding or those requiring an urgent/emergent procedure. Specific reversal agents for warfarin and dabigatran are available to expedite normal hemostasis. See Rapid Reversal below for more detail.
Procedural bleeding risk is based on the nature of the procedure itself, the urgency with which it must be performed, and practitioner skill. Patient comorbidities and medications also influence the risk of bleeding during a particular procedure.2 Many organizations provide recommendations on procedure bleed risk categorization, though these are not always consistent. Such recommendations are not meant to replace clinical judgement or negate the importance of consulting with the practitioner performing the procedure if there is uncertainty regarding the risk of bleeding. For example, Thrombosis Canada categorizes procedural bleeding risks as minimal, low/moderate, or high, whereas regional medical imaging guidelines dichotomizes procedures into low-risk and high-risk groups.1 This underscores the importance of proactively consulting with the practitioner performing the procedure to ensure appropriate and safe patient care. Refer to Figure 1: Procedure bleeding risks and Appendix A: When to Withhold Antiplatelet and Anticoagulants for Medical Imaging Procedures for more information on bleeding risks for specific procedures.
Figure 1: Procedure bleeding risks (adapted from Thrombosis Canada, 2021)1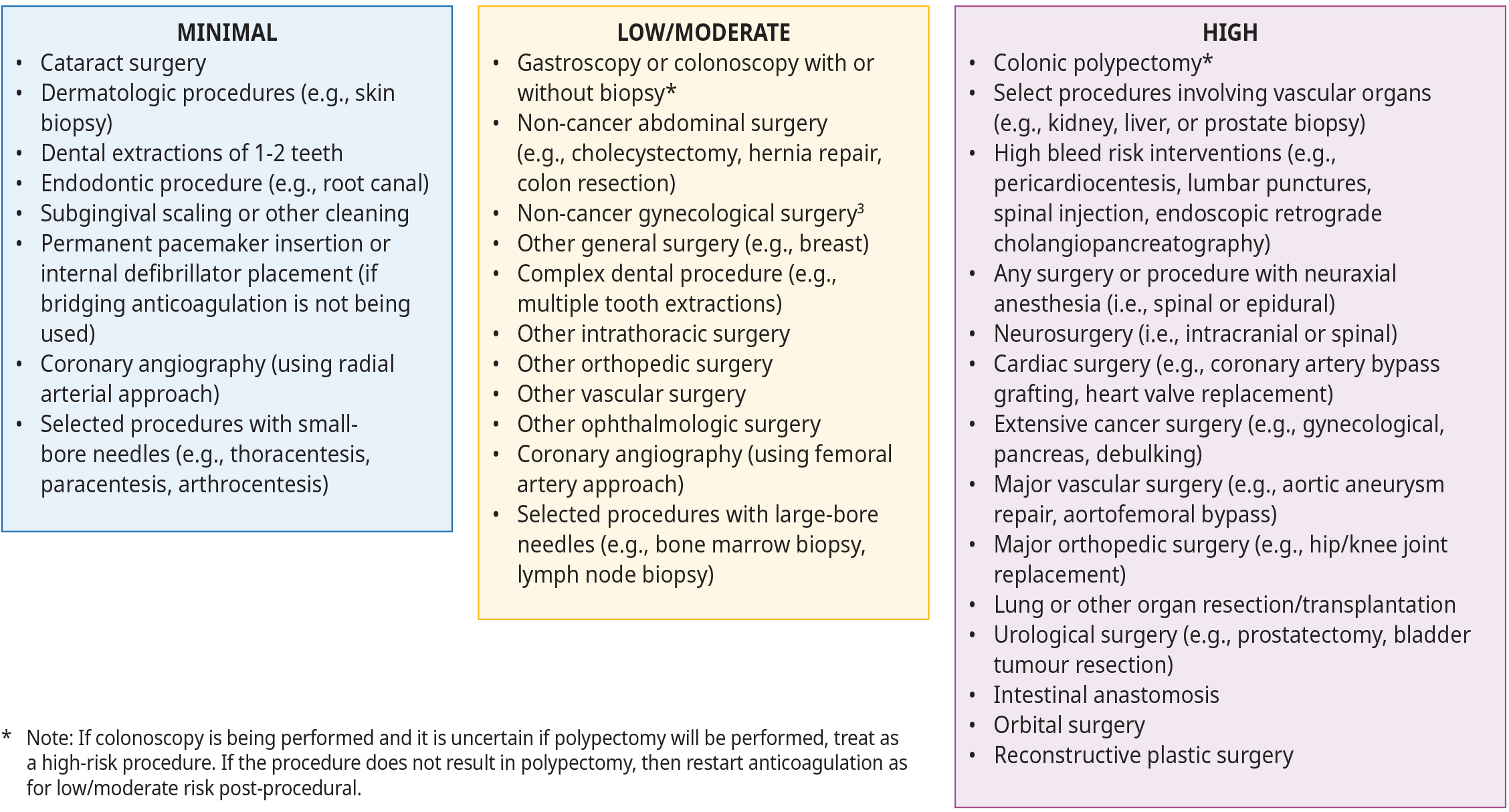
Periprocedural Management for Warfarin
Warfarin must be discontinued for several days to allow normalization of the anticoagulant effect. This might take longer in older patients and those treated to a higher target international normalized ratio (INR) (e.g., INR 3.0). Refer to Figure 2: Considerations for periprocedural management of warfarin below for information regarding how to determine the pre-procedure INR that would be considered ‘safe’ to go ahead (referred to as ‘safe INR’ throughout this document), whether bridging is required, and exact timing of drug interruption.
Special considerations for warfarin interruption in patients with epidural catheters:
- Do not start warfarin until epidural catheter is removed.
- Standard prophylactic dosing of LMWH can be used with an epidural in place.
- Do not give therapeutic level dosing of LMWH or any DOAC with an epidural catheter in place.
- Epidural catheter should not be removed within 12 hours after a dose of LMWH.
- Do not give LMWH until 4 hours after removal of epidural catheter (and there are no neurological concerns).
- Always discuss timing of epidural catheter removal with anesthesiologist.
Periprocedural Management for DOACs
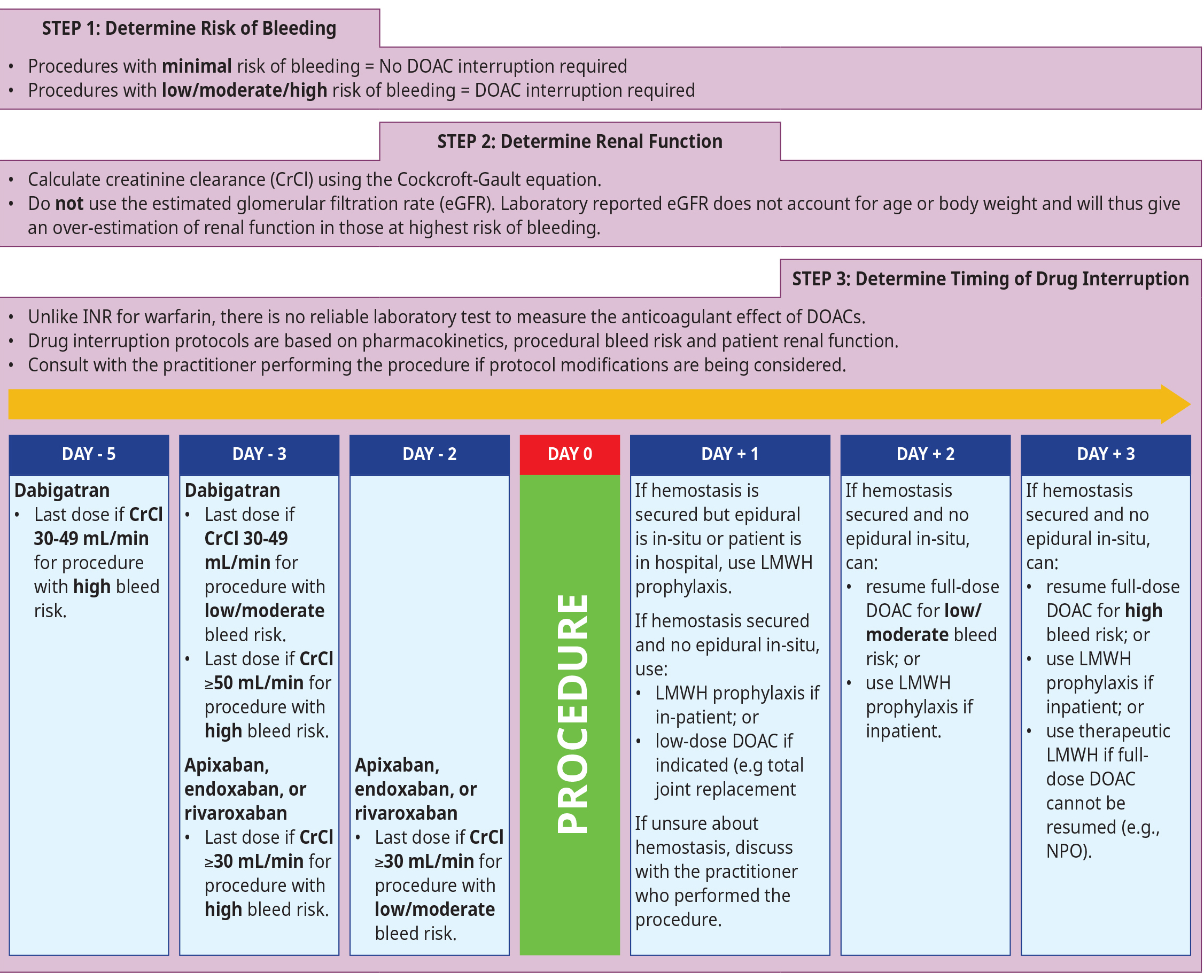
Protocols for periprocedural DOAC management have been evaluated in clinical studies and are well described in consensus recommendations and manufacturer instructions. Timing of the last dose of a DOAC primarily depends on the procedural bleeding risk, patient renal function, and drug half-life.1 Refer to Figure 3: Considerations for periprocedural management of DOACs for more details and below for additional considerations for DOAC management:
- Bridging with a fast-acting anticoagulant (e.g., LMWH) is not required for DOACs due to their rapid onset and offset of action.1,2
- Timing of the last dose of DOAC is unclear for patients with severe renal dysfunction (CrCl < 30 mL/min).1 Because clearance of the DOAC will be delayed in these patients, a longer duration off DOAC than what is recommended in Figure 3: Considerations for periprocedural management of DOACs may be required. Consult a specialist to determine when to stop DOAC prior to procedure (e.g., RACE consult line).
- For patients having neuraxial anesthesia or postoperative analgesia (i.e., epidural), some anesthesiologists prefer a longer duration of DOAC interruption than what is recommended in Figure 3: Considerations for periprocedural management of DOACs. Discuss further with an anesthesiologist.
Figure 2: Considerations for periprocedural management of warfarin
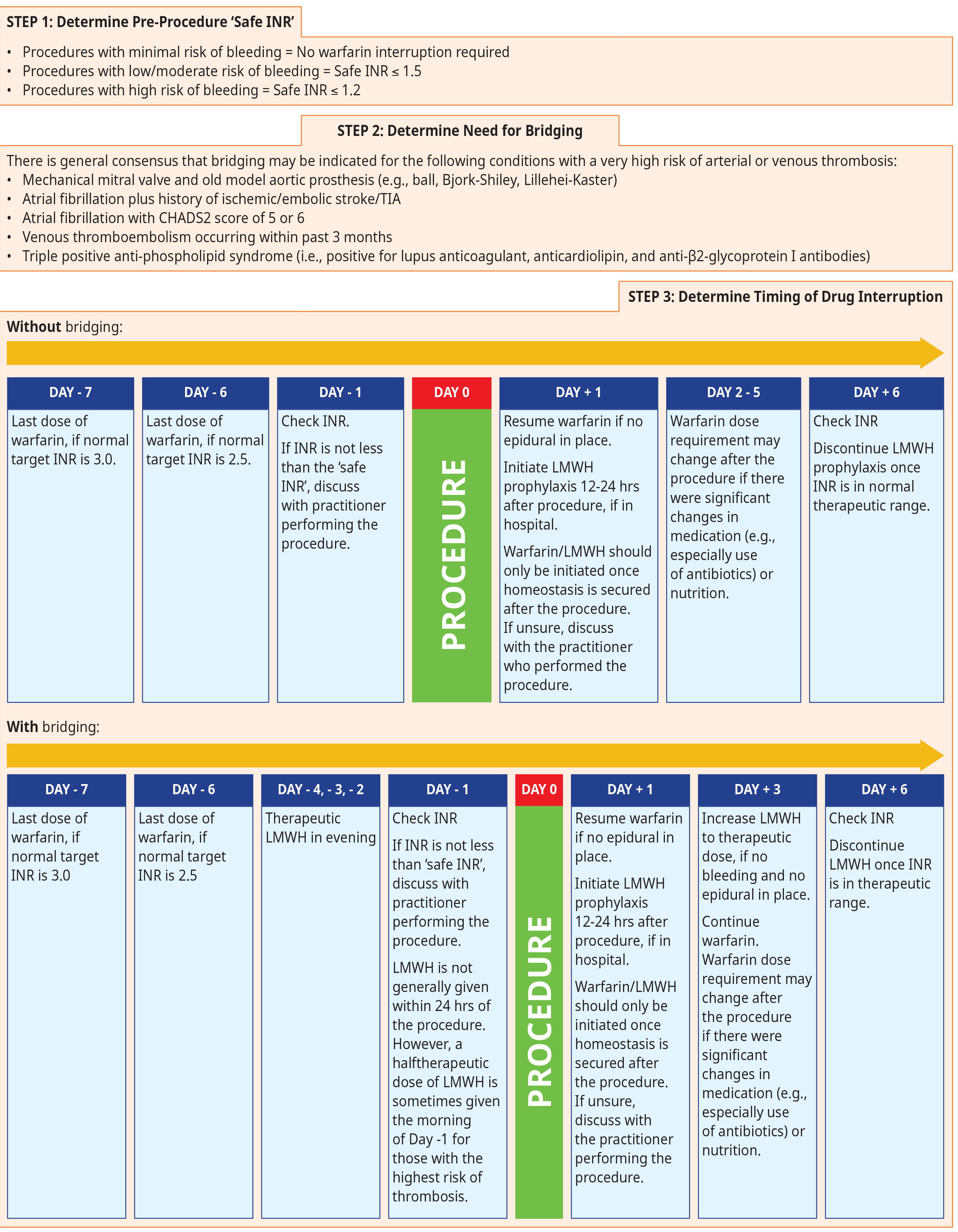
Figure 3: Considerations for periprocedural management of DOACs
Rapid Reversal for Serious Bleeding or Urgent Procedures
Bleeding is a common adverse event of all anticoagulants.8,9 Complete and rapid reversal of an OAC is necessary when a patient presents with serious bleeding or requires an urgent or emergent procedure. Individual reversal agents for warfarin and DOACs are outlined below.
Controlling the source of bleeding is important. Additional considerations in patients with serious bleeding or is requiring procedure associated with very high risk of critical bleeding (e.g., neurosurgery):12
- Maintain platelet count >50 X 109/L (>100 X 109/L if intracranial hemorrhage) with platelet transfusion.
- Consider platelet transfusion if patient on antiplatelet therapy (excluding acetylsalicylic acid).
- Consider tranexamic acid for trauma-associated coagulopathy (1 g IV given within 3 hours of trauma) or abnormal uterine bleeding (1 g PO or IV). Contraindicated in hematuria and no definitive benefit in gastrointestinal bleeding.
- Recommend fibrinogen replacement if fibrinogen <1.5 g/L.
Therapeutic Agents for Rapid Warfarin Reversal
Plasma-derived prothrombin complex concentrate (PCC) is the most effective agent for rapid reversal of warfarin.10 Effects are realized within minutes and last up to 6 hours. Vitamin K must be coadministered to provide sustained reversal.10 Refer to Appendix B: Warfarin Reversal Flow Chart for flow chart on how to rapidly reverse warfarin with a combination of PCC and vitamin K.
Plasma-derived prothrombin complex concentrate (PCC)
- Product of choice for rapid reversal of warfarin because it contains factors II, VII, IX, X.
- Is available at most hospitals and can be ordered through the blood bank.
- Has a short duration (approximately 6 hrs) so must be used concomitantly with IV vitamin K (5-10 mg). See below for additional information on vitamin K administration.
- Contains heparin and thus is contraindicated in patients with heparin-induced thrombocytopenia, liver coagulopathy, and disseminated intravascular coagulation.
- Use may be associated with clinically important thrombosis.
- Caution should be observed if PCC is administered to a pregnant patient as there is a lack of published evidence for PCC use in this population. This is especially true during the peripartum and early postpartum periods due to the elevated risk of thrombosis.
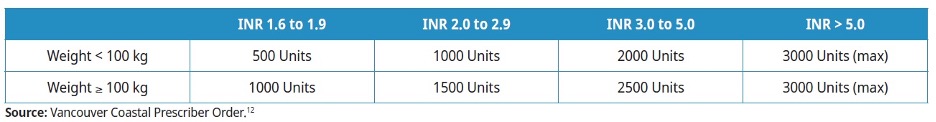
- The following PCC dosing is recommended:12
Vitamin K
- IV delivery is the fastest and most reliable way to obtain the vitamin K anticoagulant reversal effect. Avoid intramuscular and subcutaneous administration.
- If the procedure if >24 hrs away, there is no difference between using IV or oral administration.
- Effect of vitamin K on INR is observed ~8 hrs after IV and ~12 hrs after PO administration.
- For full reversal in urgent scenarios, give 5-10 mg in 50mL normal saline infused over 30 minutes. This reduces the rare risk of anaphylactoid reactions.
- A large dose of vitamin K (i.e., >5 mg) can lead to difficulty with re-anticoagulation.
Frozen Plasma
- Frozen plasma is no longer indicated for the reversal of warfarin due to lower efficacy, higher complications, and larger administration volume compared to PCC.
- Frozen plasma should still be used in massive transfusion scenarios.
Therapeutic Agents for Rapid DOAC Reversal
DOACs do not often require reversal agents because their anticoagulant effect is quickly reversed by withholding doses due to their short half-lives.11 However, rapid reversal may be indicated for life-threatening bleed situations or when an urgent or emergent procedure is required.11Currently, only dabigatran has an approved reversal agent available in Canada (i.e., idarucizumb). While andexanet alfa is effective for rapid reversal of direct factor Xa (FXa) inhibitors and has regulatory approval in some countries, it is not yet approved or available in Canada.
Idarucizumab
- Idarucizumab can only be used to reverse dabigatran. Cannot be used to reverse FXa inhibitors (i.e., apixaban, edoxaban, rivaroxaban).
- A monoclonal antibody that completely reverse the anticoagulant effect of dabigatran within minutes. The duration of action is 24 hrs in most patients.13
- Caution should be observed for patients with hereditary fructose intolerance as the drug contains 4 g of sorbitol per dose.13
- Adverse reactions: urinary tract infection, constipation, hypersensitivity reactions (e.g., bronchospasm, rash, pyrexia, pruritis).13
- Recommended dosing is 5 g IV bolus, administered as two consecutive 2.5 g doses no more than 15 min apart.14
Plasma-derived prothrombin complex concentrate (PCC)
- See Therapeutic Agents for Warfarin Reversal for PCC product details and dosing information.
- Has been used off-label for rapid reversal of DOACs for life-threatening bleeding, based on very weak evidence. No randomized trials have been published on the use of PCC as a rapid reversal agent for direct FXa inhibitors (i.e., apixaban, edoxaban, and rivaroxaban).10
- The duration of action for PCC for DOAC reversal is unclear. Concurrent administration of IV vitamin K is not required, unlike for warfarin reversal with PCC.
- PCC is available at most hospitals, though generally requires Transfusion Medicine department approval. Automatic approval for DOAC reversal if intracranial bleed and/or critical bleed may be available, depending on health authority.
- Off-label recommended one-time dose of PCC for patients with intracranial bleeding: 2000 units (weight-based dosage: 25 units per kg; max 3000 units).
References
- DOACs: Perioperative Management. Thrombosis Canada; 2021. https://thrombosiscanada.ca/clinicalguides/
- Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative Management of Patients With Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern Med. 2019;179(11):1469. doi:10.1001/jamainternmed.2019.2431
- Cheng HYHH, Cheung VYT. Patients on Long-Term Warfarin Undergo Gynecological Surgeries: A 10-Year Review of Perioperative Anticoagulation. J Obstet Gynaecol Can. 2018;40(2):199-204. doi:10.1016/j.jogc.2017.05.037
- Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-3291. doi:10.1182/bloodadvances.2018024893
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373(9):823-833. doi:10.1056/NEJMoa1501035
- Kovacs MJ, Wells PS, Anderson DR, et al. Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP2): double blind randomised controlled trial. BMJ. Published online June 9, 2021:n1205. doi:10.1136/bmj.n1205
- Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132(13):1365-1371. doi:10.1182/blood-2018-04-848333
- Vazquez S, Rondina MT. Direct oral anticoagulants (DOACs). Vasc Med. 2015;20(6):3. doi:10.1177/1358863X15600256
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood. 2008;111(10):4871-4879. doi:10.1182/blood-2007-10-120543
- Recommendations for Use of Prothrombin Complex Concentrates in Canada. National Advisory Committee on Blood and Blood Products; 2022. https://thrombosiscanada.ca/clinicalguides/
- Chan YH, Chao TF, Lee HF, et al. Different Renal Function Equations and Dosing of Direct Oral Anticoagulants in Atrial Fibrillation. JACC Asia. 2022;2(1):46-58. doi:10.1016/j.jacasi.2021.11.006
- Prescriber Order: Prothrombin Complex Concentrate (PCC). Published online 2021.
- Product Monograph Including Patient Medication Information Praxbind®. Published online April 18, 2019.
- DOACs: Management of Bleeding. Thrombosis Canada; 2021. https://thrombosiscanada.ca/clinicalguides/
Abbreviations
DOAC Direct Acting Oral Anticoagulant
LMWH Low Molecular Weight Heparin
INR International normalized ratio
IV Intravenous
PCC Prothrombin Complex Concentrate
Resources
Clinical Decision Support Tools
- Child-Pugh Calculator
- Cockcroft-Gault Calculator
- PharmaCare Special Authority: Provides benefit status for medication coverage and specific medical circumstances of coverage, depending on BC PharmaCare plan rules.
- Thrombosis Canada periprocedural algorithm tool
Consultation Supports
- RACE: Rapid Access to Consultative Expertise Program: RACE means timely telephone advice from specialist for Physicians, Medical Residents, Nurse Practitioners, Midwives, all in one phone call.
- Monday to Friday 0800 – 1700
- Online at www.raceapp.ca or though Apple or Android mobile device.
- Local Calls: 604–696–2131 | Toll Free: 1–877–696–2131
- For a complete list of current specialty services visit the Specialty Areas page.
- PathwaysBC: An online resource for current and accurate referral information for specialists and specialty clinics, including wait times and areas of expertise.
Related Guidelines
- Thrombosis Canada
- HealthLink BC
- BC Guidelines: Warfarin
- BC Guidelines: Direct Acting Oral Anticoagulants (DOACs)
- BC Guidelines: Stroke and Transient Ischemic Attack
- BC Guidelines: Atrial Fibrillation
Additional Resources
- Health Data Coalition: An online, physician-led data sharing platform that can assist you in assessing your own practice in areas such as chronic disease management or medication prescribing.
- General Practice Services Committee:
- Practice Support Program: Offers focused, accredited training sessions for BC physicians to help improve practice efficiency and support enhanced care.
- Chronic Disease Management and Complex Care Incentives: Compensates for the time and skill needed to work with patients with complex conditions or specific chronic diseases.
- Appendix A: When to withhold antiplatelet and anticoagulants for medical imaging procedures (PDF, 110KB)
- Appendix B: Flowchart for Warfarin Reversal with vitamin K and/or PCC (PDF, 93KB)
Associated Documents
The following documents accompany this guideline:
- Patient Record Sheet: Warfarin (PDF, 116KB)
- Patient Record Sheet: Warfarin Before & After Procedures (PDF, 116KB)
- Patient Record Sheet: DOAC Before & After Procedures (PDF, 116KB)
- Contributors List (PDF, 163KB)
This guideline is based on scientific evidence current as of the Effective Date.
This guideline was developed by the Guidelines and Protocols Advisory Committee, approved by the British Columbia Medical Association, and adopted by the Medical Services Commission.
The principles of the Guidelines and Protocols Advisory Committee are to:
Contact InformationGuidelines and Protocols Advisory Committee E-mail: hlth.guidelines@gov.bc.ca Web site: www.BCGuidelines.ca Disclaimer The Clinical Practice Guidelines (the “Guidelines”) have been developed by the Guidelines and Protocols Advisory Committee on behalf of the Medical Services Commission. The Guidelines are intended to give an understanding of a clinical problem, and outline one or more preferred approaches to the investigation and management of the problem. The Guidelines are not intended as a substitute for the advice or professional judgment of a health care professional, nor are they intended to be the only approach to the management of clinical problem. We cannot respond to patients or patient advocates requesting advice on issues related to medical conditions. If you need medical advice, please contact a health care professional. |