Adverse drug events (ADEs) in PharmaNet
PharmaNet displays firsthand standardized records of adverse drug events (ADEs) in acute care settings, allowing B.C. pharmacists to better evaluate prescriptions for patient safety.
ADE information is being recorded in PharmaNet by several Vancouver Coastal Health acute care sites as part of the ActionADE research trial.
ActionADE is a software application developed by B.C. researchers to document ADEs and facilitate the sharing of ADE information between health systems and care providers.
It's important for clinical users of PharmaNet information to interpret these records as ADEs, not simply as adverse drug reactions (ADRs). Previously, PharmaNet only contained ADRs and allergies, but some records will now be much broader.
For more information, visit PharmaCare Policy Manual, Section 3.19: Recording Adverse Drug Reaction and Allergy Information in PharmaNet.
On this page:
- Preventing repeat adverse drug events
- How should practitioners interpret ADE information?
- ActionADE research
- User cheat sheets by vendor
Preventing repeat adverse drug events
ADEs are the harmful and unintended consequences of, or response to, medication use. They include such things as drug interactions, dosing issues, allergies, and issues around non-adherence.
Adverse drug reactions are a subset of ADEs. They are the harmful and unintended consequence of, or response to, medication despite appropriate use (i.e., at normal doses).
Annually in B.C., ADEs result in approximately:
- 276,000 emergency department visits
- 102,000 admissions
- 4,500 deaths
- $100 million per year in health costs
Nearly one third of ADEs are repeat events, of which 75% could be prevented with stronger collaboration across the healthcare sector. The inclusion of ADE records in PharmaNet will help pharmacists readily assess drug impacts, better evaluate prescriptions for patient safety, and become key players in averting hospitalizations and deaths due to ADEs.
How practitioners should interpret *ADE information
PharmaNet still contains and permits the recording of ADRs by community pharmacists. You can recognize an ActionADE record by its unique identifier:
| *ADE_HHMM (HHMM = time ADE recorded) |
As ADEs include a variety of clinical situations beyond ADRs, the clinical decisions made when ADEs are presented to PharmaNet users must be done in the ADE context.
Example: An ADE to phenytoin where the patient presents with severe skin reaction should be treated differently than an ADE to phenytoin where the dose was subtherapeutic resulting in seizure. In the former, one might look to another medication for the patient, whereas in the latter, the practitioner should ensure the dose is adequate to control seizures.
ADE records contain more information than PharmaNet’s ADR records:
- The ADE type, certainty, outcome, frequency and dose are recorded under the suspected DIN in the ADR section of a patient’s PharmaNet profile
- The ADE symptoms are recorded in the Clinical Conditions section of a patient’s PharmaNet profile
Users must match the symptoms to the ADE details across these sections to gain full clinical insight to the observed ADE. If you see *ADE in Clinical Conditions, look to the ADR records for further information and comments.
| ADVERSE REACTION (ZPB2) | Warfarin - DIN 2240205 |
|---|---|
| Adverse Reaction Comment: *ADE_1450-Subtherapeutic Dose_Certain_Hospital extended_3mg_daily | |
| CLINICAL CONDITION (ZPB1) | *ADE_1450_Deep vein thrombosis |
| Clinical Condition Comment: INR=1 | |
*ADE_1450 – Denotes an “ADE” record and permits the linkage of symptom and ADE information.
It will be unusual for a patient to have more than one ADE recorded on their profile, however if they do, the timestamps should match for the ADR symptoms and ADE details.
Please note that as ActionADE is a research project, there will be limited patients with these new ADE records. Pharmacists can still record ADRs in PharmaNet as per the usual process.
ActionADE research
The ActionADE research team’s goals are:
- Improving ADE documentation and reporting
- Bridging information gaps across healthcare providers and between sites
- Timely alerts
- Better data for research and reporting
Through collaboration and integrated knowledge, the research team aims to achieve appropriate clinical intervention and a reduced rate of repeat ADEs observed in acute care.
Community pharmacists are asked to record their clinical decision in PharmaNet when they are presented with ADE information upon reviewing patient profiles, or after receiving ADE alerts as part of the claim submission process. User cheat sheets highlight the workflow and intervention codes that pharmacists can use to record their decisions and inform the research.
Research outcomes will be used to inform future management of ADEs and improvements to PharmaNet.
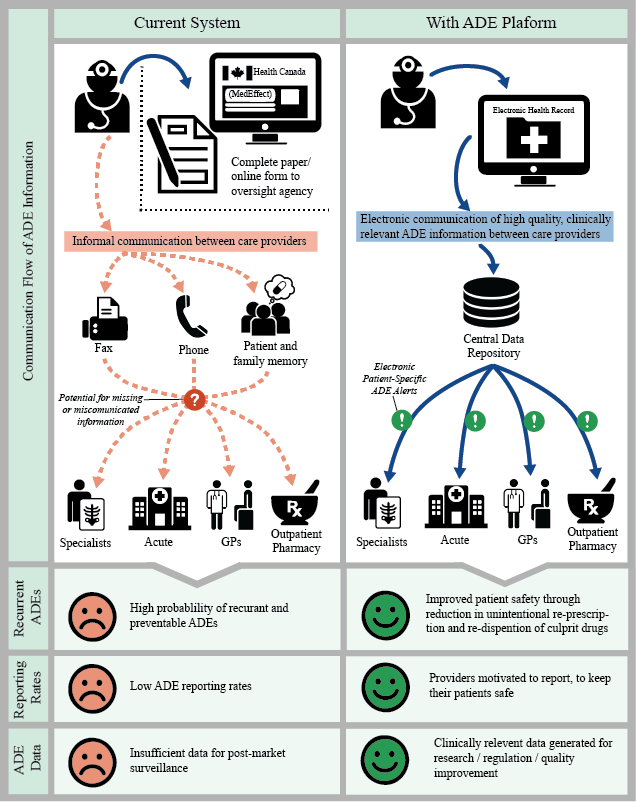
Source: Peddie et al. (2016).
User cheat sheets by vendor
These ADE cheat sheets have been included to provide clinicians with examples of how software applications will present information and record decisions in PharmaNet. Note, some software vendors have made changes to their systems to match this information together for the end user.
- Users in acute care, community practice and current version pharmacy systems (PDF, 353KB)
- Shoppers Drug Mart Users - refer to in-store web
- WinRx (Applied Robotics) Users (PDF, 683KB)
