Infectious Diarrhea - Guideline for Investigation

Revised Date: October 27, 2022
Recommendations and Topics
- Scope and Rationale
- Key Recommendations
- Diagnostic Tests for Infectious Diarrhea
- Diagnostic Approach for Acute Diarrhea
- Test Interpretation
- Persistent Diarrhea, Negative IDP
- Resources
- Associated Documents
Scope and Rationale
This document provides guidance for primary care practitioners regarding adults and children greater than 2 months of age on appropriate testing for suspected community onset infectious diarrhea, including Clostridioides difficile (formerly Clostridium difficile) infection (CDI). This guideline does not apply to outbreak situations, or patients with hospital onset diarrhea. The document includes a brief test interpretation and indications for antimicrobial management that is applicable for the general outpatient population, however it does not provide in-depth management of infectious diarrhea.
While clinical symptoms and exposure history may narrow down the possible causes of infectious diarrhea, previously there was a potential need for multiple stool tests, and healthcare visits to determine the cause. The Infectious Diarrhea Panel (IDP) is a new stool test that combines stool cultures, ova & parasites (O&P) and C. difficile. IDP detects a standardized set of 14 viral, bacterial and protozoa pathogens (Table 1: Pathogens that are included in every laboratory’s Infectious Diarrhea Panel [IDP]) within a single specimen. Not only does IDP detect a broader range of pathogens than prior methods, but it is also faster and more sensitive. It functionally replaces stool cultures and O&P; however, standalone C. difficile tests are still available. This guideline serves to describe the most appropriate use of IDP, considering that IDP is an expensive test. The guideline also describes the use and interpretation of C. difficile tests, due to advances in the understanding of CDI.
Key Recommendations
- Stool testing is not required in most cases of acute ( ≤ 7 days) diarrhea or resolving diarrhea.
- The Infectious Diarrhea Panel (IDP) should be requested if diarrhea is severe of any duration or prolonged >7 days. This is a new test that replaces stool cultures and O&P, and it also detects C. difficile.
- Request IDP only once per diarrheal episode. Only one specimen is required i.e., O&P x2 is no longer required.
- For most patients with infectious diarrhea, treatment is supportive with targeted antimicrobial management guided by the patient’s clinical history, course of illness and pathogen identified by IDP.
- If the IDP is positive but the patient is healthy and no longer symptomatic, antimicrobial treatment is not required in most cases. Antimicrobial treatment is required if Entamoeba histolytica, typhoidal Salmonella, or Vibrio cholera was detected by IDP. Antimicrobial treatment may also be warranted in those at risk of transmitting certain pathogens (e.g., Giardia spp. and Shigella spp.) to others.
- Standalone C. difficile test should be requested in those with suspected recurrence or unexpected persistence of C. difficile infection (CDI), and in patients who have been hospitalized for more than 5 days and develop nosocomial diarrhea.
- A positive C. difficile result does not differentiate between infection and colonization (i.e., asymptomatic carriage). Treatment is required only in those who are symptomatic, where C. difficile is the likely cause.
- Stop and/or avoid any antibiotics in patients with positive STEC (Shiga Toxin-producing E.coli) results, due to risk of hemolytic uremic syndrome (HUS). Patients with high-risk strains (e.g., E.coli O157:H7) require immediate assessment and may require hospitalization. See Appendix 1: Frequently asked questions (FAQ) - What is the difference between Shiga toxin-producing E.coli (STEC) and E.coli O157:H7?.
Diagnostic Tests for Infectious Diarrhea
-
Infectious Diarrhea Panel (IDP) - (New for 2022)
The Infectious Diarrhea Panel (IDP) is a new test that functionally replaces the “Stool Culture” and the “Stool for Ova & Parasites” tests. It also includes C. difficile (for patients >2 years of age), although the standalone C. difficile test is still available. Refer to Appendix 1: FAQ - Why is C. difficile included in IDP? for more information.
The IDP detects the most common enteric viral, bacterial, and protozoal pathogens (Table 1: Pathogens that are included in every laboratory's Infectious Diarrhea Panel [IDP]) faster and with greater sensitivity than culture and microscopy. IDP combines a multiple gene target (multiplex) nucleic-acid amplification test (NAAT) with other methods. When applicable, antimicrobial susceptibilities are provided.
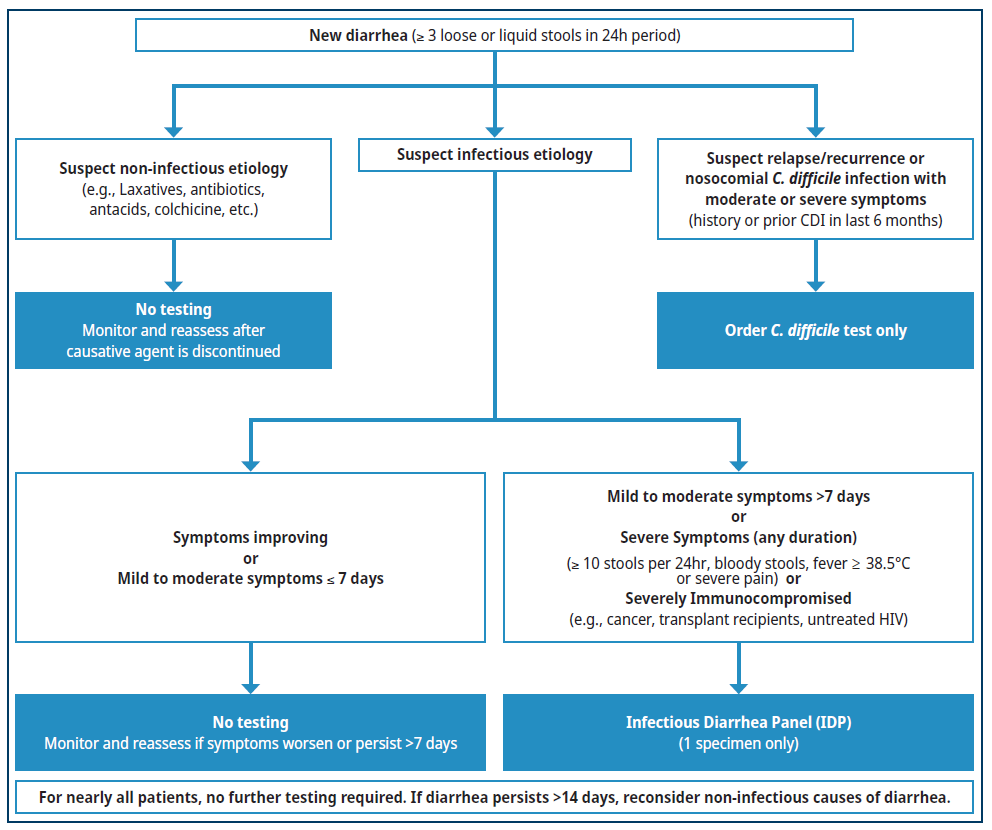
Figure 1. Diagnostic workup of acute diarrhea
A stool microscopy test for protozoa may be requested if the IDP is negative and the patient:
- Has a history of recent travel or immigration from low- or middle-income country; OR
- Is severely immunocompromised
Consider consultation with a specialist to assess likelihood of rare or atypical infections.
Refer to Appendix 1: FAQ – Considering that the management of most infectious diarrhea is supportive, what is the rationale for performing diagnostic testing for diarrhea? for more information.
Table 1: Pathogens that are included in every laboratory's Infectious Diarrhea Panel (IDP)a
| Viral Pathogen | Bacterial Pathogen | Protozoal Pathogen |
|---|---|---|
| Adenovirus 40/41 Norovirus GI/GII Rotavirus |
Campylobacter spp. |
Cyclospora cayetanensis |
aThe list of pathogens may be modified periodically, in line with changes in epidemiology and technology
bC. difficile is not routinely reported in those under 2 years old
Refer to Appendix 1: FAQ – How was the list of pathogens in Table 1 established? for more information.
- Test ordering for Infectious Diarrhea Panel (IDP):
- If either stool culture or O&P is ordered, if available the laboratories will automatically perform the IDP.
- Only one specimen is required. Except in rare or specific circumstances this test should not be repeated.
- Specimen collection and storage for IDP will be as determined by your local laboratory provider:
- Advise patients that samples should be submitted to the laboratory as soon as possible. If more than a 2-hour delay is anticipated that, store refrigerated at 4ºC.
Clostridioides difficile (formerly Clostridium difficile) Test
This is the most common pathogen detected in all stool specimens in British Columbia (BC). However, a positive test does not differentiate C.difficile infection from colonization. Colonization is defined as the absence of clinical symptoms of C. difficile infection (e.g., diarrhea, ileus, toxic megacolon) or the presence of an alternative explanation of these symptoms.1 C. difficile colonization does not require treatment. Refer to Appendix 1: FAQ - How do I discern C. difficile infection from colonization? for more information.
- Test ordering for Clostridioides difficile:
- If IDP, stool culture or O&P are added on the same requisition, only the IDP will be performed.
- If only C. difficile is required, specifically order “C. difficile” on the requisition.
- Do not routinely order on patients ≤ 24 months old as they are likely to have asymptomatic colonization. Consult with a medical microbiologist or a pediatric infectious diseases physician.
- Tests should not be repeated within 7 days unless the test is negative and there is a clinical suspicion of C. difficile infection.
- Tests should not be performed in patients who are on C. difficile treatment, e.g., oral vancomycin, metronidazole, or fidaxomicin.
- Test of cure should not be performed.
- Specimen collection and storage for C. difficile:
- Collection and storage as determined by your local laboratory provider.
Stool Microscopy for Protozoa
Certain rare protozoal pathogens are not detectable by the IDP, and detection requires manual microscopy. Such pathogens are not endemic within Canada and are usually acquired after recent (within 6 months) travel out of country with consumption of contaminated foods/liquids. Stool microscopy should not be ordered for routine diarrhea testing.
- Test ordering for Protozoa:
- Manually write “Stool Microscopy” on the requisition.
- Only one specimen is usually required. If high suspicion, consider ordering two specimens at least one day apart.
See Appendix 1: FAQ – Why are helminths (worms) not included in IDP? for more information.
- Specimen collection and storage for Protozoa:
- Collect stool in container with "SAF Fixative” or as determined by your local laboratory provider.
Stool Culture
This test is being phased out and integrated into the IDP.
Stool for O&P
This test is being phased out and integrated into the IDP.
Other related Laboratory Tests
Several diagnostic tests are available for gastrointestinal infections that may not cause diarrhea (e.g., worm infections, (refer to Appendix 1: FAQ- I'm still concerned about a worm infection. What do I order to make a diagnosis?). or for non-infectious causes of diarrhea (e.g., inflammatory bowel disease). See Appendix 2: Other related laboratory tests for more information
Diagnostic Approach for Acute Diarrhea
Clinical History and Evaluation
Diarrhea is defined as the passage of three or more loose or liquid stools per 24 hours OR more frequent than is normal for an individual.2 Clinical history is essential prior to testing, and should include:3
- Assessment of disease severity and duration. Severe Diarrhea (of any duration) is defined as diarrhea with one or more of the following:
- Fever ≥38.5°C.
- More than 10 loose to watery bowel movements in 24 hours.
- Severe abdominal pain.
- Blood in stools.
- Hospitalization due to diarrhea.
- Suspected Hemolytic uremic syndrome (HUS) (e.g., anemia, hypertension, acute kidney injury or neurologic symptoms in children*).4
- Assessment of risk factors and underlying disease(s):
- Immunocompromised is defined as having the immune response attenuated by the administration of immunosuppressive therapy, malnutrition or by some disease processes (e.g., untreated Human Immunodeficiency Virus [HIV] Infection or congenital immunodeficiency).5
- People at extremes of age and/or with severe frailty have increased risk of dehydration due to diarrhea.
- Assessment for non-infectious causes of diarrhea:
- Recent use of new medication or change in dose/frequency of current medication (e.g., laxatives)
- Other common causes: changes in diet, non-infectious gastrointestinal diseases, and endocrine disorders.
Refer to Table 3: Causes of non-infectious diarrhea for more information.
- Prior history of or risk for C. difficile including:
- Previous history or suspected current episode of C. difficile infection.
- Antibiotic use within 3 months prior to onset of diarrhea.
- Hospitalization or visit to a healthcare facility within 30 days prior to onset of diarrhea.
- Long-term care facility resident.
- Assessment of suspected exposures and potential sources of infectious diarrhea:
- Travel to or immigration from low/middle income countries.
- Consumption of contaminated and/or undercooked food.
Consumption of or swimming in contaminated water (including oceans and natural bodies of water). If Mild to Moderate Acute Diarrhea
- Less than or equal to 7 days duration:
- No testing required.
- Most cases resolve with supportive treatment (e.g., electrolytes and hydration) within 7 days.6
- More than 7 days duration:
- Order an Infectious Diarrhea Panel (IDP).
- While most viral and bacterial gastroenteritis in immunocompetent patient resolves within 7 days, occasionally these pathogens result in prolonged diarrhea or severe symptoms. In contrast, protozoal pathogens such as Giardia may result in several weeks to months of diarrhea and malabsorption. The goal of timely diagnosis is to decrease morbidity, when possible, with targeted treatment as indicated and to provide prognosis. See Appendix 1: FAQ – Considering that the management of most infectious diarrhea is supportive, what is the rationale for performing diagnostic testing for diarrhea?
If Severe Diarrhea (of any duration) or Immunocompromised Patient
- Order an Infectious Diarrhea Panel (IDP).
- The goal of timely diagnosis is to decrease morbidity with targeted treatment and to provide prognosis. Refer to Appendix 1: FAQ – Considering that the management of most infectious diarrhea is supportive, what is the rationale for performing diagnostic testing for diarrhea? for more information.
Risk for C.difficile Infection Only
- With the availability of IDP (which also detects C. difficile), a standalone C. difficile test has limited indications:
- Patients with symptoms similar to prior C. difficile infection where relapse or recurrence is suspected; or
- Patients who develop nosocomial diarrhea, after being hospitalized for more than 5 days.
- Traditionally, C. difficile test was only ordered in patients with new onset diarrhea after recent antibiotics and/or hospitalization. However, C. difficile infection can occur in the absence of these risk factors. The IDP will be able to detect these unsuspected community-acquired casers.
- Do not order a C. difficile test unless the patient has diarrhea. Formed stools should NOT be submitted for testing.
- Testing for colonization or test of cure should not be ordered as a positive result may lead to unnecessary treatment due to high rates of asymptomatic colonization. Refer to Appendix 1: FAQ – How do I discern C. difficile infection from colonization?
 TOP
TOP
Test Interpretation
Infectious Diarrhea Panel
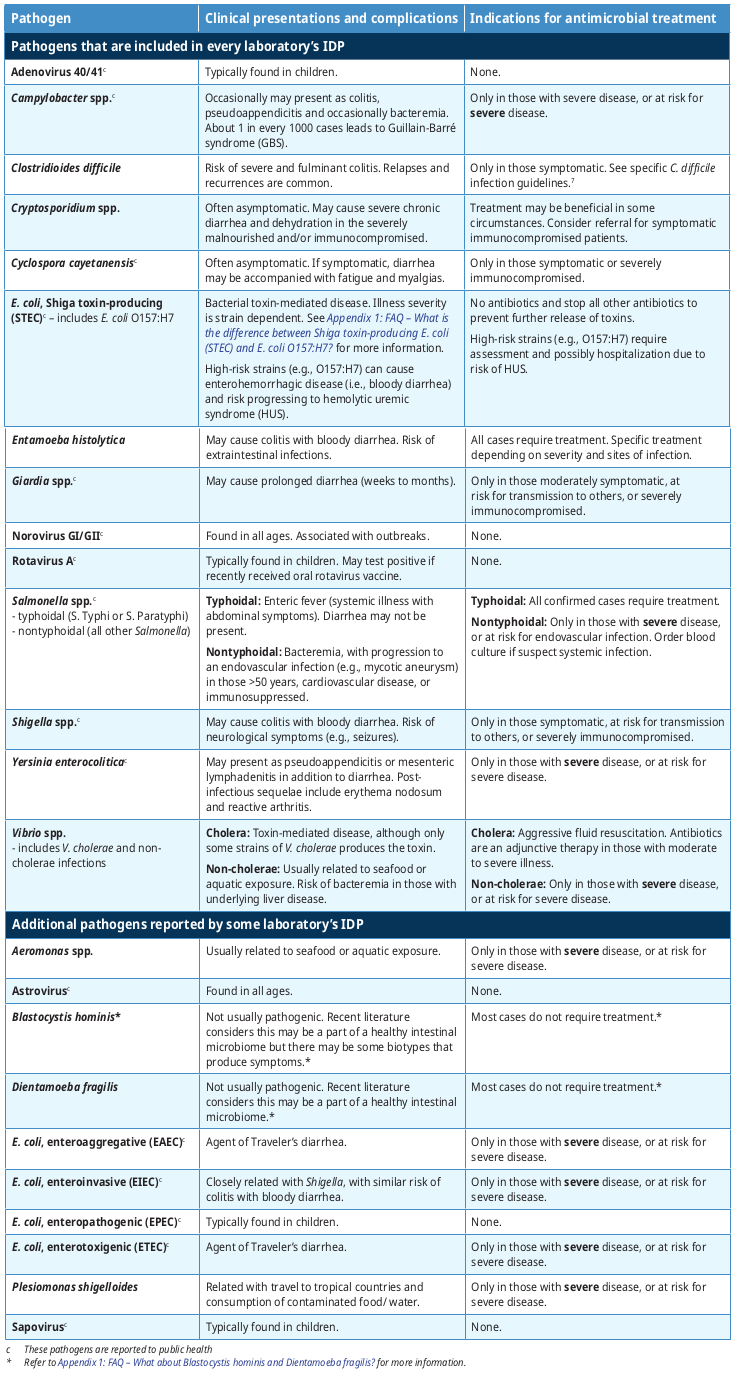
Table 2: Summary of clinical presentation and indications for antimicrobial treatment summarizes the most common clinical presentations and complications for pathogens reported by the IDP. Note that all BC laboratories will always detect a minimal set of 14 pathogens (see Table 1: Pathogens that are included in every laboratory’s Infectious Diarrhea Panel [IDP]), although some BC laboratories may report additional pathogens.
Most diarrhea is self-limited and managed by supportive treatment. Table 2: Summary of clinical presentation and indications for antimicrobial treatment briefly provides guidance when antimicrobial treatment is indicated (or avoided) for the general population with typical infections. Patients with complicated medical histories, atypical presentations and/or severe symptoms may require specific treatment and/or specialist consultation. Refer to Appendix 1: FAQ – The syndromic IDP test appears to oppose principles of test stewardship, where diagnostic tests should be judiciously selected based on the differential diagnosis. What was the rationale in adopting this approach? for more information.
Table 2: Summary of clinical presentation and indications for antimicrobial treatment
Limitations of the Infectious Diarrhea Panel (IDP)
1.Detection of resolved or asymptomatic infections:
-
The IDP is very sensitive at detecting low amounts of pathogens. It may also detect non-viable pathogens that represent resolved infections, or asymptomatic infection/colonization.
- Typically for immunocompetent patients, if the IDP is positive and the symptoms have resolved or are improving, no further action is required, with a few exceptions (see Table 2: Summary of clinical presentation and indications for antimicrobial treatment). (i.e., Shiga toxin-producing E. coli [STEC], Entamoeba histolytica, typhoidal Salmonella, Vibrio cholerae).
2.Susceptibility testing for bacterial pathogens is not always performed:
- If a bacterial pathogen is detected by the IDP, the laboratory will attempt to provide additional testing for confirmation and susceptibility testing, if indicated.
- Occasionally, susceptibility testing may not be successful as the organism may not be cultivatable. If antimicrobial treatment is warranted, refer to the reporting laboratory’s antibiogram or discuss with the laboratory physician/ medical microbiologist.
3.Other pathogens and bacterial toxins are not detected:
- While IDP detects a broader range of pathogens than traditional methods, it does not detect all causes of infections diarrhea (Refer to Appendix 1: FAQ – How was the list of pathogens in Table 1 established?). For example, it does not detect Bacillus cereus, S. aureus enterotoxin and other toxin-mediated disease. However, such infections are typically brief and do not warrant further investigation.
4.Potential false positive results:
- False positive results are rare. However, if the pathogen identified does not correlate clinically, please contact the laboratory physician/medical microbiologist for further discussion.
5.If multiple pathogens are detected, the interpretation can be challenging:
- This often occurs in those with a travel history with ingestion of contaminated foods/water.
- Those with severe symptoms or at risk of severe disease could be treated where applicable.
- In those with mild to moderate symptoms, address the pathogens which are more likely to cause severe disease (i.e., C. difficile, Entamoeba histolytica, Giardia, Shigella, typhoidal Salmonella, Vibrio cholerae).
- Consider consultation with a specialist for management of complex infections.
C.difficile
C. difficile test results must always be interpreted in clinical context, as patients may be colonized in the presence of diarrhea due to other causes (e.g., laxatives, tube feeds, irritable bowel syndrome, viral infection).
To facilitate differentiation of infection from colonization (asymptomatic carriage), some laboratories test for the toxin protein itself, as the presence of the toxin protein suggests that infection is more likely than colonization. Laboratories that detect both the toxin protein and toxin gene may report the test as positive, negative or indeterminate (see Appendix 1: FAQ – How do I discern C. difficile infection from colonization?). Indeterminate results correlate with colonization, although infection is possible. Clinical correlation with the patient’s symptoms is essential to differentiate C. difficile infection from colonization.7 In many instances treatment can be avoided.
Public Health Notification
Laboratories directly notify the associated Public Health unit of all reportable enteric pathogens whenever identified. However, the most responsible physician needs to notify the Public Health Unit/Medical Health Officer who may be involved for the following:
- Food handlers
- Daycare employees and children who attend daycare or elementary school
- Health care workers with direct patient contact in long-term and acute-care facilities
- Potential outbreaks where food or water has been identified as a possible source
- Patients identified as part of a community or facility outbreak
In these scenarios, the Public Health Unit/Medical Health Officer will manage stool testing in the affected case, and possibly their contacts.
Persistent Diarrhea, Negative IDP
If initial testing for infectious diarrhea is non-diagnostic and the patient has unresolving symptoms, clinical re-evaluation to delineate other causes of diarrhea should be undertaken. Typically, if the IDP is negative and the diarrhea has persisted for greater than 14 days, then the diarrhea likely has a non-infectious etiology (see Table 3: Causes of non-infectious diarrhea).
Repeat testing with the same IDP panel is not likely to be useful. However, patients with certain risk factors may have rare causes of infectious diarrhea.
Severely Immunocompromised
- Severely immunocompromised (e.g., uncontrolled HIV, transplant recipients, current chemotherapy, hematological malignancies. Refer to specialist for evaluation and possibly specialized testing.
Travel and Immigrant Populations
The following patient may have an infection with a pathogen that is not on the IDP:
- Recent travel, immigrants and refugees within 6 months from low to middle income countries.
In such cases, order a Stool Microscopy Exam for Protozoa by manually writing “Stool Microscopy” on the requisition. Selective stool culture for additional bacterial pathogens not in the IDP may also be available. Contact your local laboratory physician/medical microbiologist to determine what additional testing might be indicated.
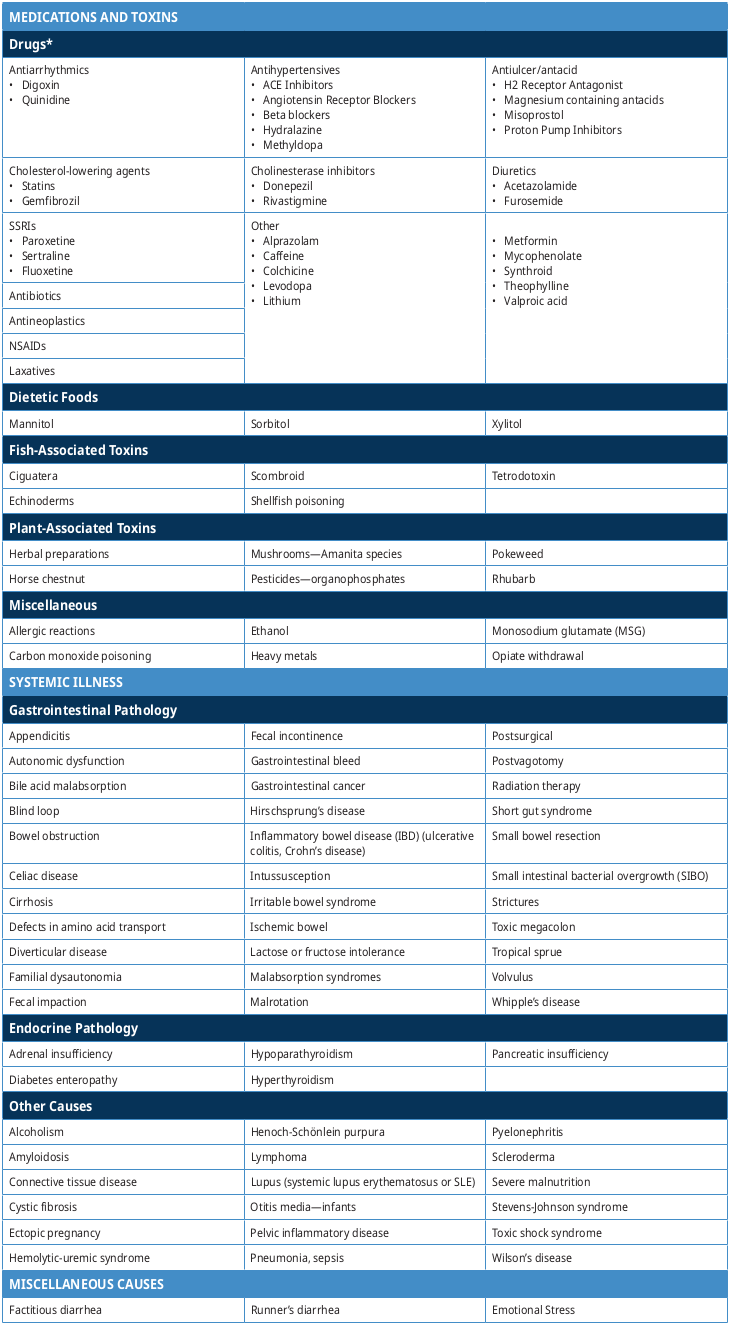
Non-Infectious Causes of Diarrhea
Table 3: Causes of non-infectious diarrhea (adapted from Lazarciuc 2018 and UpToDate)8, 9
*Many drugs can cause diarrhea; the following list is not exhaustive but contains some examples of drugs commonly associated with causing diarrhea.
Resources
References
- Crobach MJT, Vernon JJ, Loo VG, Kong LY, Péchiné S, Wilcox MH, et al. Understanding Clostridium difficile Colonization. Clin Microbiol Rev [Internet]. 2018 Apr [cited 2021 Sep 1];31(2). Available from: https://journals.asm.org/doi/10.1128/CMR.00021-17
- World Heath Organization. Diarrhoeal disease [Internet]. World Heath Organization; 2017 [cited 2021 Sep 1]. Available from: https://www.who.int/news-room/ fact-sheets/detail/diarrhoeal-disease
- Toward Optimized Practice (TOP) Working Group for Microbiology. Ordering Stool Test for Investigation of Suspected Infectious Diarrhea [Internet]. Accelerating Change Transformation Team; 2014 [cited 2021 Sep 1]. Available from: https://actt.albertadoctors.org/CPGs/Lists/CPGDocumentList/infectious- diarrhea-guideline.pdf
- Gerber A, Karch H, Allerberger F, Verweyen HM, Zimmerhackl LB. Clinical Course and the Role of Shiga Toxin–Producing Escherichia coli Infection in the Hemolytic‐Uremic Syndrome in Pediatric Patients, 1997–2000, in Germany and Austria: A Prospective Study. J Infect Dis. 2002 Aug 15;186(4):493–500.
- BC Centre for Disease Control. Communicable Disease Control Manual [Internet]. BC Centre for Disease Control; 2019 [cited 2021 Sep 1]. Available from: http:// www.bccdc.ca/health-professionals/clinical-resources/communicable-disease-control-manual
- Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017 Nov 29;65(12):e45–80.
- Johnson S, Lavergne V, Skinner AM, Gonzales-Luna AJ, Garey KW, Kelly CP, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin Infect Dis. 2021 Sep 7;73(5):e1029–44.
- Nicole Lazarciuc. Diarrhea. In: Rosen’s emergency medicine: concepts and clinical practice [Internet]. Ninth edition. Philadelphia, PA: Elsevier; 2018 [cited 2021 Sep 1]. Available from: https://books.google.ca/books?id=OANODgAAQBAJ&pg=PA249&lpg=PA249&dq=Diarrhea.+Nicole+Lazarciuc+PERSPECTIVE.+Epidemiol ogy+DIAGNOSTIC+APPROACH.+Pathophysiology.+Differential+Diagnosis+Considerations&source=bl&ots=WyrVHFw_qM&sig=ACfU3U0qaKnUvveFmbWTPgvEiPRO5CVggQ&hl=en&sa=X&ved=2ahUKEwjG99znqt7yAhU1FTQIHXzpCD4Q6AF6BAgDEAM#v=onepage&q=Diarrhea.%20Nicole%20Lazarciuc%20PERSPECTIVE.
%20Epidemiology%20DIAGNOSTIC%20APPROACH.%20Pathophysiology.%20Differential%20Diagnosis%20Considerations&f=false - Bonis P, Lamont T. Approach to the adult with chronic diarrhea in resource-rich settings. uptodate [Internet]. 2017; Available from: https://www.uptodate.com/ contents/approach-to-the-adult-with-chronic-diarrhea-in-resource-rich-settings
- Wendy Barr, Andrew Smith. Acute Diarrhea in Adults. Am Fam Physician. 2014 Feb 1;89(3):180–9.
- Torres-Miranda D, Akselrod H, Karsner R, Secco A, Silva-Cantillo D, Siegel MO, et al. Use of BioFire FilmArray gastrointestinal PCR panel associated with reductions in antibiotic use, time to optimal antibiotics, and length of stay. BMC Gastroenterol. 2020 Dec;20(1):246.
- Bateman AC, Kim YJ, Guaracao AI, Mason JL, Klos RF, Warshauer DM. Performance and Impact of the BioFire FilmArray Gastrointestinal Panel on a Large Cyclospora Outbreak in Wisconsin, 2018. J Clin Microbiol. 2020 Jan 28;58(2):e01415-19.
- Machiels JD, Cremers AJH, van Bergen-Verkuyten MCGT, Paardekoper-Strijbosch SJM, Frijns KCJ, Wertheim HFL, et al. Impact of the BioFire FilmArray gastrointestinal panel on patient care and infection control. PloS One. 2020;15(2):e0228596.
- Schreckenberger PC, McAdam AJ. Point-Counterpoint: Large Multiplex PCR Panels Should Be First-Line Tests for Detection of Respiratory and Intestinal Pathogens. Caliendo AM, editor. J Clin Microbiol. 2015 Oct;53(10):3110–5.
- Al-Jumaili IJ, Shibley M, Lishman AH, Record CO. Incidence and origin of Clostridium difficile in neonates. J Clin Microbiol. 1984 Jan;19(1):77–8.
- Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The Association Between Idiopathic Hemolytic Uremic Syndrome and Infection by Verotoxin- Producing Escherichia coli. J Infect Dis. 1985 May 1;151(5):775–82.
- Mah J, Lieu A, Holmes E, Vaughan S. A case of disseminated strongyloidiasis after multiple courses of immunosuppression. Can Med Assoc J. 2022 Jan 24;194(3):E89–92.
- BC Centre for Disease Control. Sushi Safety [Internet]. BC Centre for Disease Control; 2016 [cited 2021 Nov 9]. Available from: http://www.bccdc.ca/resource- gallery/Documents/Educational%20Materials/EH/FPS/Fish/SushiSafety.pdf
- Parto N, Caturay A. Evidence brief: control of parasites by freezing in fish for raw consumption [Internet]. Public Health Ontario; 2017 [cited 2021 Nov 9]. Available from: https://www.publichealthontario.ca/-/media/documents/E/2017/eb-raw-fish-parasites.pdf
Abbreviations
- CDI C. difficile infection
- EAEC E. coli, enteroaggregative
- EIA Enzyme immunoassay
- EHEC E. coli, enterohemorrhagic
- EIEC E. coli, enteroinvasive
- EPEC E. coli, enteropathogenic
- ETEC E. coli, enterotoxigenic
- GBS Guillain-Barré syndrome
- HIV Human Immunodeficiency Virus
- HUS Hemolytic Uremic Syndrome
- IBD Inflammatory bowel disease
- IDP Infectious Diarrhea Panel
- MSG Monosodium glutamate
- NAAT Nucleic-acid amplification test
- NSAIDs Nonsteroidal anti-inflammatory drugs
- STEC Shiga toxin-producing E. coli
Practitioner Resources
- RACE: Rapid Access to Consultative Expertise Program – www.raceconnect.ca
RACE means timely telephone advice from specialist for Physicians, Medical Residents, Nurse Practitioners, Midwives, all in one phone call.
Monday to Friday 0800 – 1700Online at www.raceapp.ca or though Apple or Android mobile device. For more information on how to download RACE mobile applications, please visit www.raceconnect.ca/race-app/
Local Calls: 604–696–2131 | Toll Free: 1–877–696–2131
For a complete list of current specialty services visit the Specialty Areas page.
If you do not receive a call-back within two hours of your request, please contact: RACE@providencehealth.bc.ca or call 604-696-2131 (Press 0)
All unanswered requests will be followed up. -
Pathways
An online resource that allows family physicians and nurse practitioners and their office staff to quickly access current and accurate referral information, including wait times and areas of expertise, for specialists and specialty clinics. See https://pathwaysbc.ca/login
-
Management of Infectious Diarrhea
- 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clinical Infectious Diseases, Volume 65, Issue 12, 15 December 2017, Pages e45–e80
- ACG Clinical Guideline: Diagnosis, Treatment, and Prevention of Acute Diarrheal Infections in Adults. American Journal of Gastroenterology: May 2016 – Volume 111 – Issue 5 – p 602–622
-
Management of C. difficile Infection
-
Functional and IBS Diarrhea
-
Travel-Related Diarrhea
-
Bugs & Drugs:
Patient, Family and Caregiver resources
- HealthLink BC: www.healthlinkbc.ca
Diagnostic Code: 009.2 (Infectious Diarrhea)
Associated documents
This guideline is based on scientific evidence current as of the effective date.
This guideline was developed by the Guidelines and Protocols Advisory Committee in collaboration with the Provincial Laboratory Medicine Services, and adopted under the Medical Services Act and the Laboratory Services Act.
For more information about how BC Guidelines are developed, refer to the GPAC Handbook available at
BCGuidelines.ca: GPAC Handbook.
THE GUIDELINES AND PROTOCOLS ADVISORY COMMITTEE
|
The principles of the Guidelines and Protocols Advisory Committee are to:
Contact Information: Guidelines and Protocols Advisory Committee PO Box Email: hlth.guidelines@gov.bc.ca Disclaimer The Clinical Practice Guidelines (the “Guidelines”) have been developed by the Guidelines and Protocols Advisory Committee on behalf of the Medical Services Commission. The Guidelines are intended to give an understanding of a clinical problem, and outline one or more preferred approaches to the investigation and management of the problem. The Guidelines are not intended as a substitute for the advice or professional judgment of a health care professional, nor are they intended to be the only approach to the management of clinical problem. We cannot respond to patients or patient advocates requesting advice on issues related to medical conditions. If you need medical advice, please contact a health care professional. |