Water quality parameters

On this page
Water quality parameters
Water quality parameters measure the physical and chemical characteristics of a water body for a specific use.
The water quality parameters included in the analysis are:
Alkalinity
Alkalinity is a measure of the water’s ability to neutralize acids.
Water with high alkalinity will experience less of a change in acidity when an acid is added to the water (CCME, 2003).
Chloride
Chloride is widely distributed in nature, mostly in the form of sodium and potassium salts.
The greatest amount of chloride is found in oceans. Chloride is an essential element for aquatic life. However, it's detrimental to aquatic organisms at high concentrations.
The application of road salt is the primary source of chloride to the environment in B.C. (Nagpal, 2003).
Organic carbon
Organic carbon is a measure of the natural organic matter dissolved in water.
Natural organic matter is typically from microorganisms and decaying plant and animal matter. It's measured as total, dissolved or particulate organic carbon.
Human sources and activities of organic carbon include:
- Municipal wastewater
- Septic systems
- Agricultural runoff
- Industrial discharges and deforestation
Organic carbon can bind to some fractions of metals and reduce their toxicity (Moore, 1998).
Dissolved oxygen
All aquatic organisms need dissolved oxygen for respiration. Oxygen enters water from the atmosphere and from photosynthesis in aquatic plants.
Flowing water contains higher dissolved oxygen concentrations than standing water because more water is circulated to the surface where it absorbs oxygen from the air. Dissolved oxygen is also affected by temperature.
Cold water can hold more oxygen than warm water. Microbes use dissolved oxygen to decompose organic matter and high levels of organic matter can lead to low oxygen in streams.
Low dissolved oxygen levels can lead to increased mortality of fish eggs and severely stress or kill aquatic life (BC ENV, 1997).
A decreasing trend in dissolved oxygen can lead to deteriorating water quality conditions.
Fluoride
Fluoride is found in most bedrock. It's leached from bedrock by groundwater and ends up in surface water and the ocean. Human sources of fluoride in the environment include industries, such as aluminum smelting and fertilizer production, and wastewater treatment plants.
At higher concentrations, fluoride accumulates in the exoskeletons and/or bone tissues of aquatic organisms and interferes with protein production. Freshwater organisms and fish are more sensitive to fluoride than marine organisms.
Fluoride becomes more toxic with increasing temperatures and less toxic with hardness (BC ENV, 2011).
Hardness
Hardness is the amount of ions in the water, primarily calcium and magnesium ions.
Natural sources of hardness are sedimentary rocks and runoff from soils. While hardness does not directly affect aquatic life, it influences the toxicity of many metals and some ions.
Certain metals and ions such as lead, zinc, copper, cadmium, nickel and sulphate become more toxic at lower concentrations of water hardness (CCME, 2003).
Metals
Metals occur naturally in the environment, leaching into water from rocks and soil.
Living organisms need varying amounts of metals such as iron, copper, manganese, molybdenum, selenium and zinc to survive.
However, these metals and others become toxic to aquatic organisms at high concentrations. Other sources of metals in water include wastewater, mining and industrial activity.
The toxicity and bioavailability of many metals depends on their oxidation state and form.
Generally, dissolved metals are more bioavailable than metals bound to sediment or in complexes with other molecules (CCME, 2003).
Nitrogen
Nitrogen is essential for many biological processes in plants and animals.
Nitrate, nitrite and ammonia are the major biologically available compounds occurring in surface water. Natural sources of nitrogen are from weathering of soil and rocks.
Nitrogen pollution is typically from municipal wastewater, agricultural run-off and groundwater.
Excessive nitrogen leads to proliferation of algae and rooted plants, lowering the oxygen content in the water and suffocating fish and other aquatic life through a process called eutrophication (Nordin, 1986).
Total suspended solids
Total suspended solids (non-filterable residue) are a measure of the suspended particulate matter in water.
Fine sediments can clog gill structures in aquatic organisms, cause injury to eye and gill membranes by abrasion and inhibit egg development.
Soil erosion from agricultural and urban run-off, removal of riparian vegetation, channelization and dredging can cause high levels of suspended sediments (BC ENV, 1997).
pH
pH is the measure of the hydrogen ion activity in a solution. This is how acidic or basic water is.
Geology of the watershed typically influences the pH of a river system.
pH affects the solubility (the amount that can be dissolved in water) and bioavailability (amount that can be used by aquatic life) of other chemical constituents in water such as metals or nutrients.
For example, metals become more toxic at lower pH and ammonia is more toxic at higher pH (CCME, 2003).
Phosphorus
Plants and animals need phosphorus to grow. It's naturally occurring in rocks, animal waste, plant material and in the atmosphere.
Phosphorus itself is not toxic in the aquatic environment. However, high levels of phosphorus will increase vegetation and algae growth causing an imbalance of production versus consumption in an ecosystem.
This process is called eutrophication and can eventually lead to decreased dissolved oxygen levels, suffocation of fish and other aquatic life.
Human activities such as the discharge of sewage effluent and run-off from residential and agricultural lands cause eutrophication (CCME, 2004).
Specific conductance
Specific conductance is the measure of water’s ability to conduct electricity at a specific temperature.
Water conducts electricity because of the salts that have dissolved in it. In the natural environment, water bodies tend to have a relatively constant range of conductivity.
However, human activity can increase the amount of dissolved salts in the water resulting in decreased health of aquatic ecosystems (USEPA, 2016).
Sulphate
Sulphate is the oxidized form of sulphur, which is essential for many biological processes in plants and animals. However, at high concentrations sulphate is harmful to aquatic organisms.
Sulphur occurs naturally in rocks as sulphides. In the presence of water, sulphide is oxidized to form sulphate.
Human sources include waste from industries that produce or use sulphates or sulphuric acid such as mining and smelting operations, kraft pulp and paper mills, textile mills, tanneries, agricultural run-off and sewage (Meays, 2013).
Turbidity
Turbidity is a measure of the lack of clarity in water.
Clay, silt, organic and inorganic matter, plankton and microorganisms can all cause water to be turbid.
In aquatic ecosystems, high turbidity can affect light penetration through the water, reducing plant growth, and smothering benthic habitats (BC ENV, 1997).
Water temperature
Water temperature affects the physical, chemical and biological properties of a water body.
Warmer water increases the rate of chemical reactions, causing more minerals or chemicals to dissolve and while it holds less gas from the atmosphere, like oxygen and nitrogen.
Aquatic organisms and fish have temperature ranges that they prefer to live in, so temperature directs the types of organisms that can live in rivers and lakes. For example, salmon in British Columbia are cold water dependent and sensitive to temperature changes.
Organisms that are exposed to temperatures outside this range are stressed, leading to stunted development and disease or with extreme changes, death. Smelters, refineries, pulp and paper mills, hydroelectric power and wastewater treatment plants can discharge warm waters to rivers and lakes.
Human activities that change landscape such as forestry, mining, dams, increased roads and parking lots can also increase water temperatures (Oliver and Fidler, 2001).
References
References
British Columbia Ministry of Environment (BC ENV). 1997. Ambient Water Quality Guidelines (Criteria) for Turbidity, Suspended and Benthic Sediments. Technical Appendix. Approved Water Quality Guidelines. Ministry of Environment, Province of British Columbia (BC ENV). https://www2.gov.bc.ca/assets/gov/environment/air-land-water/water/waterquality/water-quality-guidelines/approved-wqgs/turbitity-or.pdf. Accessed May 2020.
British Columbia Ministry of Environment (BC ENV). 1997. Water Quality: Ambient Water Quality Guidelines for Dissolved Oxygen. Technical Appendix. Approved Water Quality Guidelines. Ministry of Environment, Province of British Columbia (BC ENV). https://www2.gov.bc.ca/assets/gov/environment/air-land-water/water/waterquality/water-quality-guidelines/approved-wqgs/dissolvedoxygen-tech.pdf. Accessed May 2020.
British Columbia Ministry of Environment (BC ENV). 2011. Water Quality: Ambient Water Quality Criteria for Fluoride. Ministry of Environment, Province of British Columbia (BC ENV). Approved water quality guidelines - Province of British Columbia. Accessed July 2025.
Canadian Council of Ministers of the Environment. 2003. Canadian water quality guidelines for the protection of aquatic life: Guidance on the Site-Specific Application of Water Quality Guidelines in Canada: Procedures for Deriving Numerical Water Quality Objectives. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, Winnipeg.
Canadian Council of Ministers of the Environment. 2004. Canadian water quality guidelines for the protection of aquatic life: Phosphorus: Canadian Guidance Framework for the Management of Freshwater Systems. In: Canadian environmental quality guidelines, 2004, Canadian Council of Ministers of the Environment, Winnipeg.
Meays, C., Nordin, R. 2013. Ambient Water Quality Guidelines for Sulphate. Technical Appendix. Approved Water Quality Guidelines. Ministry of Environment, Province of British Columbia (BC ENV). https://www2.gov.bc.ca/assets/gov/environment/air-land-water/water/waterquality/water-quality-guidelines/approved-wqgs/sulphate/bc_moe_wqg_sulphate.pdf. Accessed May 2020.
Moore, D. 1998. Ambient Water Quality Criteria for Organic Carbon in British Columbia. Approved Water Quality Guidelines. Ministry of Environment, Province of British Columbia (BC ENV). Approved water quality guidelines - Province of British Columbia. Accessed July 2025.
Nagpal, N.K. 2003. Ambient water quality guidelines for chloride. Approved Water Quality Guidelines. Ministry of Environment, Province of British Columbia (BC ENV). https://www2.gov.bc.ca/assets/gov/environment/air-land-water/water/waterquality/water-quality-guidelines/approved-wqgs/chloride-or.pdf. Accessed May 2020.
Nordin, R.N., Pommen, L.W. 1986. Water Quality Criteria for Nitrogen (Nitrate, Nitrite, and Ammonia). Technical Appendix. Approved Water Quality Guidelines. Ministry of Environment, Province of British Columbia (BC ENV). https://www2.gov.bc.ca/assets/gov/environment/air-land-water/water/waterquality/water-quality-guidelines/approved-wqgs/nitrogen-amonia-tech.pdf. Accessed May 2020.
Oliver, G., Fidler, L. 2001. Towards a Water Quality Guideline for Temperature in the Province of British Columbia. Approved Water Quality Guidelines. Ministry of Environment, Province of British Columbia (BC ENV). Approved water quality guidelines - Province of British Columbia. Accessed July 2025.
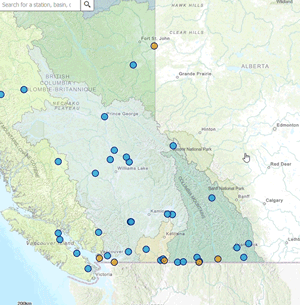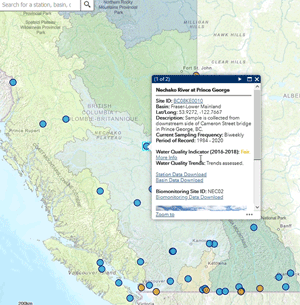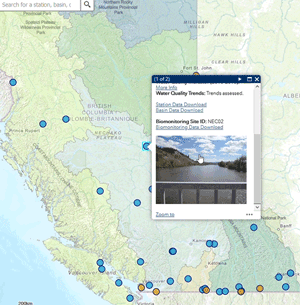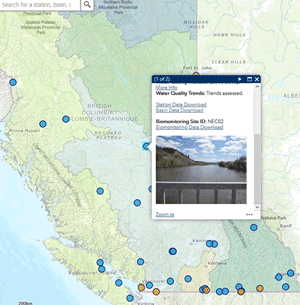
Use our interactive map to explore monitoring stations in the Canada-B.C. Water Quality Monitoring Program.