PharmaCare Newsletter
January 2026 PharmaCare Newsletter
On this page:
Find past issues on the newsletter search page.

Q: What is the role of Duavive in the management of menopause symptoms?
A: The answer is in the current edition of PAD Refills. Make sure to subscribe so you don’t miss out on news and updates!
Adverse reactions and clinical conditions in PharmaNet patient profiles
PharmaCare is reminding pharmacists to enter only clinical information about a client’s allergies and clinical conditions in PharmaNet, and not related notes about the client.
Clients will receive this information when they request their PharmaNet profile, and related notes entered by the pharmacy may be confusing or even detrimental to their care. It is important to upload allergy and clinical information to PharmaNet, but related notes about the client should be recorded only in your local system.
To request the removal of an incorrect entry in the Adverse Reaction or Clinical Condition field on a PharmaNet profile, please complete the HLTH 5550 - Request to Inactivate Adverse Reaction/Clinical Condition on PharmaNet Profile (PDF, 1MB), and fax to the PharmaNet Data Quality Services Team at 250-953-0486.
Resources
- HLTH 5550 - Request to Inactivate Adverse Reaction/Clinical Condition on PharmaNet Profile (PDF, 1MB)
- PharmaNet Data Quality Services Team
- Email: PharmaNet@gov.bc.ca
- Fax: 250-953-0486
New P&O coverage maximum based on Canadian pricing
Effective January 1, 2026, PharmaCare will cover prosthetic and orthotic (P&O) components up to the cost of the lowest-priced component available from a Canadian supplier. The pricing policy update supports buy-Canadian initiatives, and responds to challenges when a “lowest-cost equivalent” component is only available through an international supplier.
PharmaCare previously funded the lowest-cost component available, regardless of supplier location. Now, when the same or similar component is offered at different prices by two or more suppliers, PharmaCare will cover the cost of the lowest-priced component from a Canadian supplier. If a component is not available from a Canadian supplier, then PharmaCare will usually cover the lowest available price amount.
This change informs only the maximum that PharmaCare will cover for a component; PharmaCare will continue to fund components from non-Canadian suppliers. However, PharmaCare strongly encourages prosthetist and orthotist providers to order components from Canadian suppliers where possible.
For more information, visit the Prosthetic and Orthotic Policy Manual.
Resources
PharmaCare now accepts P&O prescriptions from NPs
PharmaCare now accepts prescriptions from nurse practitioners (NPs) to support applications for coverage of new and upgraded prosthetic and orthotic (P&O) devices and supplies.
NPs must be licensed in B.C. and knowledgeable about prostheses or orthoses. BC College of Nurses and Midwives (BCCNM) and the Ministry of Health’s Professional Regulation Branch have confirmed that prescribing prostheses and orthoses falls within an NP’s scope of practice.
The NP’s name and BCCNM license number can be entered as the referring practitioner information on PharmaCare coverage applications and payment invoices.
In some cases, PharmaCare requires a written recommendation from a specialist or multi-disciplinary team to consider coverage. For full details, refer to Prosthetic and Orthotic Policy Manual, Section 5.1.4: Devices requiring an assessment by a multi-disciplinary team or specialist physician.
For more information, visit the Prosthetic and Orthotic Policy Manual and Forms for Medical Device Providers.
Resources
Reminder: Include PHN on SA forms
The PharmaCare Special Authority (SA) team is receiving many forms that are missing the patient’s Personal Health Number (PHN). The team is reminding prescribers to include all necessary information when submitting SA forms.
SA forms with missing information are returned to the prescriber to complete and re-submit, which causes delays in coverage.
Prescribers are also encouraged to use SA eForms to submit requests, as the turnaround time is often quicker than faxed forms.
Resources
BC PharmaCare wishes you a Happy New Year!
Policy Spotlight: Drug administration fee
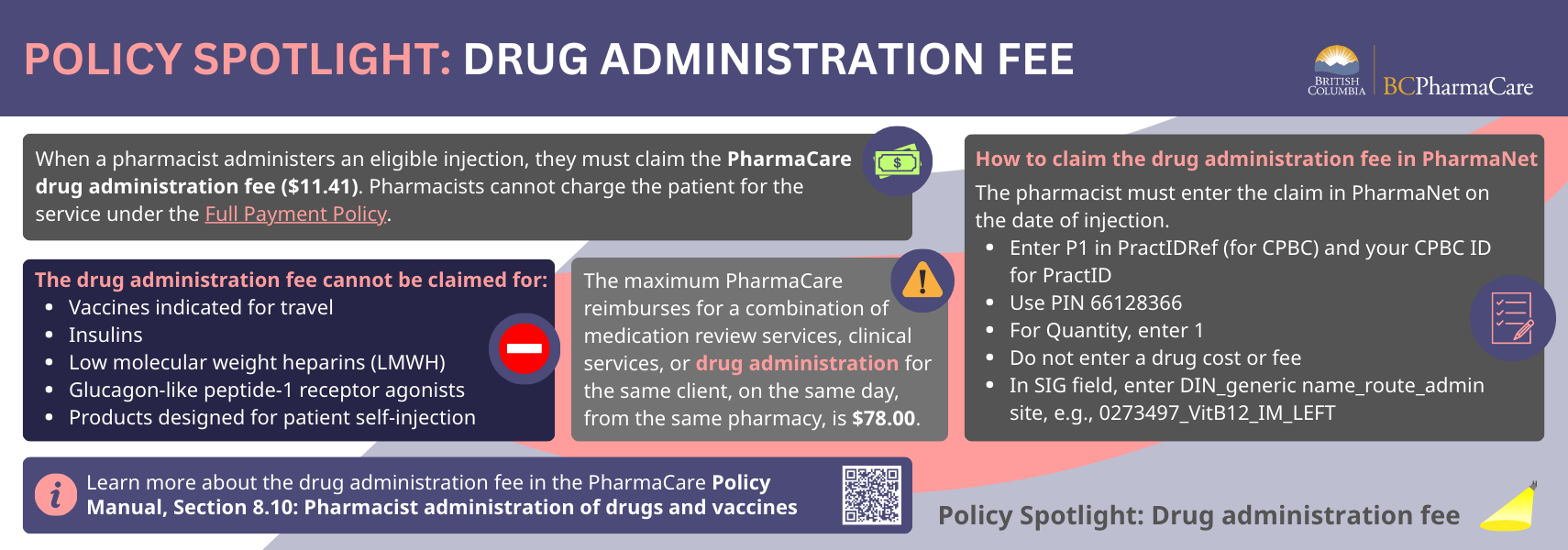
Resources
- Drug administration fee
- PharmaCare Policy Manual, Section 8.10: Pharmacist Administration of Drugs and Vaccines
- PharmaCare Policy Manual, Section 5.10: Full Payment Policy
RAT kits available free of charge until supplies run out
Pharmacies are encouraged to order RAT kits from distributors, available free of charge until supplies run out. Pharmacy distributors have stockpiled kits to be available to pharmacies and stores until the supply is exhausted or expired (i.e., late 2026).
2026 new year reminders
2026 provider payment schedule
The weekly and monthly provider payment schedule for 2026 is available on the Information for pharmacies webpage. Pharmacies should note that payments may be remitted earlier than dates indicated in the schedule. Published dates remain the authorized payment dates and should be the guide for claims-related accounting.
Blood glucose test strip annual quantity limits
On January 1, 2026, clients were assigned their annual limit of blood glucose test strips (BGTS). Beginning January 1, please ensure you use the regular BGTS PINs for claims until clients exceed their annual limit.
All strips purchased by a client, regardless of the payer, count toward the client’s annual limit.
BGTS information for clients
Information for the public is available online about annual quantity limits for BGTS and in the printable information sheet, Blood glucose test strips – annual limits (PDF, 167KB). (Translations are available in 15 languages on the PharmaCare information sheets webpage).
- Blood glucose testing – annual limits, criteria for additional strips, for health professionals
- BGTS PINs
- Blood glucose test strips – patient information sheet (PDF, 167KB)
- Annual quantity limits for BGTS – webpage for the public
Fair PharmaCare annual deductibles reset in the new year
On January 1, 2026, PharmaNet was updated with 2026 annual deductible and family maximum amounts. Deductible accumulations will be reset to zero.
Fair PharmaCare coverage levels for 2026 are based on a family’s 2024 net income. Income from Universal Child Care Benefits and Registered Disability Savings Plans and some BC Housing subsidies are not included when determining coverage levels.
Deductible information for patients
This can be a stressful time for Fair PharmaCare beneficiaries as many are paying the full cost of their drugs. Please let them know that they may be eligible for PharmaCare’s monthly deductible payment option. Once they register, PharmaCare immediately pays for 70% of their eligible drug costs. Fair PharmaCare registrants can get information about their deductible and family maximum by:
- Requesting a confirmation of Fair PharmaCare coverage letter or
- Calling us Monday to Friday, 8 am to 8 pm and Saturdays 8 am to 4 pm from the Lower Mainland at 604-683-7151 or from the rest of B.C., toll-free, at 1-800-663-7100
Fair PharmaCare information sheet in 15 languages
It’s a great time of year to print copies of the Fair PharmaCare information sheet to have on hand. The information sheet explains in plain language Fair PharmaCare deductibles, eligible costs, the monthly payment option and more. You may also want to post the QR codes for PharmaCare information sheets for clients. They are available in 15 languages.

Formulary and listing updates
Limited Coverage benefits: aripiprazole (Abilify Asimtufii®), belumosudil (Rezurock™), mylife™ YpsoPump® Starter Kit
PharmaCare has added the following limited coverage items to the PharmaCare drug list. Special Authority approval is required for coverage.
| Drug name | aripiprazole (Abilify Asimtufii®) | ||
|---|---|---|---|
| Date | December 17, 2025 | ||
| Indication | Management of the manifestations of schizophrenia or related psychotic disorders (not dementia-related) | ||
| DINs | 02554569 02554577 |
Strength & form | 720 mg/2.4 mL suspension in a pre-filled syringe for intramuscular injection 960 mg/3.2 mL suspension in a pre-filled syringe for intramuscular injection |
| Notes | Patients with existing Special Authority coverage for aripiprazole injection (Abilify Maintena®) will automatically receive Special Authority coverage for Abilify Asimtufii | ||
| Drug name | belumosudil (Rezurock™) | ||
|---|---|---|---|
| Date | December 17, 2025 | ||
| Indication | For the treatment of patients aged 12 years and older with chronic graft-versus-host-disease (cGvHD) after failure of at least two prior lines of systemic therapy | ||
| DIN | 02526115 | Strength & form | 200 mg oral tablet |
| Notes | Belumosudil has been added to the high-cost drugs list with an allowed markup of 1% | ||
mylife™ YpsoPump® Starter Kit
Effective December 17, 2025, Ypsomed AG’s mylife YpsoPump Starter Kit has been added as a limited coverage benefit. The mylife YpsoPump Starter Kit replaces the existing YpsoPump Starter Kit. The existing infusion sets and reservoirs are compatible with the mylife YpsoPump.
A new PIN has been created for the mylife YpsoPump Starter Kit (PIN 45230021). The PINs for the Ypsomed infusion sets and reservoirs remain the same. All PINs for starter kits, infusion sets and reservoirs can be found at Diabetes Product Identification Numbers (PINs).
The mylife YpsoPump can be paired with the Dexcom G6 Continuous Glucose Monitor and, when used with the mylife CamAPS FX mobile application, creates a hybrid-closed loop system that offers automated insulin delivery. This is the first HCL insulin delivery system with PharmaCare coverage.
The mylife YpsoPump Starter Kit will be available to PharmaCare-covered patients at an eligible cost of $7,000, subject to their PharmaCare plan rules (e.g., annual deductibles and family maximums). The updated prices of infusion sets and reservoirs are listed in the table below.
| Product | PIN | Previous price per unit | New price per unit | Effective date of new price |
|---|---|---|---|---|
| YpsoPump® Orbit soft | 46340034 | $10.00 | $10.50 | December 17, 2025 |
| YpsoPump Inset® | 46340035 | $10.00 | $10.50 | |
| YpsoPump® Orbit micro | 46340036 | $10.00 | $10.50 | |
| YpsoPump® Reservoir | 47450009 | $6.70 | $5.50 | February 6, 2026 |
| Drug name | mylife™ YpsoPump® Starter Kit | |||
|---|---|---|---|---|
| Date | December 17, 2025 | |||
| Indication | For the management of diabetes mellitus | |||
| DIN | 45230021 | Strength & form | Insulin pump | |
| Notes | Dexcom G6 (PharmaCare Benefit) |
Compatible insulins | Admelog®, Apidra® (PharmaCare Benefits) NovoRapid® and Humalog® (Non-Benefit |
|
Non-benefits: trofinetide (Daybue®), semaglutide (Wegovy®)
PharmaCare has decided not to cover the following drugs for the noted indications.
| Drug name | trofinetide (Daybue®) | ||
|---|---|---|---|
| Date | December 1, 2025 | ||
| Indication | For the treatment of Rett syndrome (RTT) in adults and pediatric patients 2 years of age and older and weighing at least 9 kg | ||
| DIN | 02552523 | Strength & form | 200 mg/mL oral solution |
| Drug name | semaglutide (Wegovy®) | ||
|---|---|---|---|
| Date | December 18, 2025 | ||
| Indication | As an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in adult patients with a body mass index (BMI) 27 kg/m2 or greater and established cardiovascular disease (CVD), defined as myocardial infarction (MI), prior stroke, or peripheral artery disease (PAD) | ||
| DINs | 02528509 02528517 02528525 02528533 02528541 |
Strength & form | 0.25 mg pre-filled pen 0.5 mg pre-filled pen 1 mg pre-filled pen 1.7 mg pre-filled pen 2.4 mg pre-filled pen |
PharmaCare is unable to list Wegovy because the manufacturer, Novo Nordisk, has stated that they are no longer seeking public coverage of Wegovy in Canada. The Pan-Canadian Pharmaceutical Alliance (pCPA) concluded negotiations without agreement, citing that the manufacturer declined to negotiate.
The Ministry of Health will review manufacturer submissions for generic brands of semaglutide for all approved indications when they are available on the market.
Discontinuation: Levemir® Penfill®, epinephrine bitartrate (Emerade®), epinephrine (Allerject®), Novolin® insulin products
Levemir® Penfill®
Novo Nordisk Canada Inc. has discontinued the insulin detemir product Levemir® Penfill®, effective December 31, 2025. As of February 28, 2026, Levemir Penfill will become a PharmaCare non-benefit.
Patients currently using Levemir Penfill should make an appointment with their prescriber to discuss switching to an alternative insulin product. Pharmacists can also help patients switch by adapting an existing Levemir prescription to another, similar insulin product by therapeutic substitution.
Patients with existing Special Authority (SA) coverage for Levemir automatically have SA coverage for insulin glargine (Basaglar®, Semglee®). Coverage does not need to be renewed. For patients covered under Plan W, Basaglar and Semglee are regular benefits.
| Drug name | insulin detemir (Levemir® Penfill®) | ||
|---|---|---|---|
| Discontinuation date | December 31, 2025 | ||
| Drug class | Basal insulin | ||
| DIN | 02271842 | Strength & form | 100 U/mL, solution for subcutaneous injection in a 3 mL cartridge |
Epinephrine bitartrate (Emerade®), epinephrine (Allerject®)
As of February 6, 2026, epinephrine bitartrate (Emerade®) and epinephrine (Allerject®) will become PharmaCare non-benefits, as these products are being discontinued.
| Drug name | epinephrine bitartrate (Emerade®) | ||
|---|---|---|---|
| Drug class | Sympathomimetics | ||
| DIN | 02458446 02458454 |
Strength & form | 0.3 mg/0.3 mL pre-filled pen 0.5 mg/0.5 mL pre-filled pen |
| Drug name | epinephrine (Allerject®) | ||
|---|---|---|---|
| Drug class | Sympathomimetics | ||
| DIN | 02382059 02382067 |
Strength & form | 0.15 mg/0.15 mL single-use autoinjector 0.3 mg/0.3 mL single-use autoinjector |
Novolin® insulin products
As described in the November 2025 PharmaCare Newsletter (PDF, 605KB) , Novo Nordisk has discontinued the following Novolin® insulin products. Refer to the table below for alternate treatment options; all are PharmaCare regular benefits. For any questions about interchangeability or therapeutic substitution of insulin products, pharmacists can email the College of Pharmacists of BC practice support at practicesupport@bcpharmacists.org.
| Discontinued product | Active ingredient | Form | DIN | Alternative treatment options |
|---|---|---|---|---|
| Novolin®ge NPH Penfill® | isophane (NPH) insulin human | 3 mL cartridge | 02024268 | Novolin®ge NPH 10 ml vial or Humulin® N cartridge |
| Novolin®ge Toronto Penfill® | insulin regular human | 3 mL cartridge | 02024284 | Novolin®ge Toronto 10 ml vial or Humulin® R cartridge |
| Novolin®ge 30/70 Penfill® | insulin regular human/isophane (NPH) insulin human 30/70 | 3 mL cartridge | 02025248 | Novolin®ge 30/70 10 ml vial or Humulin® 30/70 cartridge |
Price reduction: filgrastim (Nypozi and Grastofil®), nirmatrelvir/ritonavir (Paxlovid®)
Effective February 6, 2026, the prices for the following products will be reduced. Prices include 8% markup.
| Drug name | Nypozi | Strength & form | Current price per unit ($) | Reduced price per unit ($) | |
|---|---|---|---|---|---|
| Date | February 6, 2026 | ||||
| DINs | 02521008 | 480 mcg/0.8 mL pre-filled syringe | 239.3971 | 182.6142 | |
| 02520990 | 300 mcg/0.5 mL pre-filled syringe | 149.6201 | 114.1342 | ||
| Drug name | Grastofil® | Strength & form | Current price per unit ($) | Reduced price per unit ($) | |
|---|---|---|---|---|---|
| Date | February 6, 2026 | ||||
| DINs | 02454548 | 480 mcg/0.8 mL pre-filled syringe | 239.3928 | 182.6142 | |
| 02441489 | 300 mcg/0.5 mL pre-filled syringe | 149.6210 | 114.1342 | ||
| Drug name | Paxlovid® | Strength & form | Current price per unit ($) | Reduced price per unit ($) | |
|---|---|---|---|---|---|
| Date | February 6, 2026 | ||||
| DIN | 02527804 | 150-100 mg tablet per dose (renal dosing package) | 1,391.9904 | 626.4000 | |
| Note | The price adjustment applies to the renal dose package of Paxlovid only. The price of the 300-100 mg dose package (DIN 02524031) remains unchanged | ||||
Your Voice: Input needed for drug decisions
The knowledge and experience of patients, caregivers and patient groups is integral to B.C.'s drug review process. If you know someone who is taking one of the drugs below or who has a condition any of the drugs treat, please encourage them to visit www.gov.bc.ca/BCyourvoice.
Your Voice is now accepting input on the following drugs:
| Drug | Indication | Input window | ||
| maralixibat (Livmarli®) | Progressive familial intrahepatic cholestasis (PFIC) in patients 3 months of age and older | December 31 to January 27 at 11:59 pm | ||
| avacincaptad pegol (Izervay™) | Non-foveal geographic atrophy (GA) secondary to age-related macular degeneration (AMD) | December 31 to January 27 at 11:59 pm | ||
| lecanemab (Leqembi®) | Adult patients with Alzheimer's disease | December 31 to January 27 at 11:59 pm | ||
| seladelpar (Lyvdelzi®) | Primary biliary cholangitis (PBC) in adults | December 31 to January 27 at 11:59 pm | ||
| palopegteriparatide (TBC) | Chronic hypoparathyroidism (hypoPT) in adults | December 31 to January 27 at 11:59 pm | ||
| epinephrine nasal spray (neffy™) | Emergency treatment of type I allergic reactions, including anaphylaxis, in adult and pediatric patients | December 31 to January 27 at 11:59 pm | ||
| vamorolone (Agamree®) | Duchenne muscular dystrophy (DMD) in patients 4 years of age and older | December 31 to January 27 at 11:59 pm | ||
| nipocalimab (TBC) | Generalized myasthenia gravis (gMG) in adult and adolescent patients | December 31 to January 27 at 11:59 pm | ||


December 24 update: PA witness fee – Eligibility, PharmaNet codes, witness log
As announced in the December 2025 PharmaCare Newsletter (PDF, 714KB), the Ministry is working on a new system to pay pharmacies a fee for witnessing doses of prescribed alternatives (PAs).
To be eligible for a PA witness fee:
- "SA" must be written on the prescription by the prescriber. If "SA" is missing, the pharmacist can confirm with the prescriber, then enter the associated PA intervention code in PharmaNet
- The drug must be on the PA drug list
- The drug must be ingested orally or applied topically (includes applying/removing fentanyl patch)
PAs witnessed in a community pharmacy must be witnessed by a pharmacist.
PAs witnessed as part of a delivery or outreach model of care (i.e., delivered directly to the client) must be witnessed by a regulated health professional (e.g., pharmacist, nurse).
PAs witnessed in a clinic by a regulated health professional are not eligible for the PharmaCare witness fee.
For full details on eligible witnessed doses of PAs, refer to the PharmaCare Policy Manual, Section 8.16: Prescribed Alternatives Witnessing Fee.
PharmaNet notation
All doses of PAs witnessed in a community pharmacy or delivered to a client must be notated with "VS" intervention code.
All non-witnessed doses of PAs dispensed in a community pharmacy must be notated with "SA" intervention code (for "safer alternative").
New intervention code for PAs witnessed in a clinic
When dispensing PAs to be witnessed in a clinic, pharmacies must use the new PharmaNet intervention code "AW – PA sent for witnessing by other health professional". PharmaCare does not pay pharmacies for witnessing that takes place in a clinic.
For full PharmaNet entry instructions, refer to PharmaCare Policy Manual, Section 8.16: Prescribed Alternatives Witnessing Fee.
PA witness log requirements
As of December 4, each witnessed dose of PA must be recorded in a witness accountability log to claim the PharmaCare witness fee. The log must include:
- Date and time drug was dispensed and witnessed
- Prescription or transaction number
- Quantity of drug witnessed
- Quantity provided as unwitnessed doses (“carries”), if any
- Total quantity dispensed
- Client signature
- Signature or initials of witnessing pharmacist (community pharmacy) or witnessing regulated health professional (delivery or outreach model of care)
The PA witness log is for PharmaCare auditing purposes and is not required when PharmaCare does not pay a witness fee. This includes situations when:
- PAs are dispensed to a clinic for witnessing by a regulated health professional
- PA drugs are not eligible for the witness fee (i.e., drugs not on PA drug list)
- Prescriber has indicated that PA doses are to be unwitnessed
The log is similar to the methadone witness log but includes the time the PA dose was witnessed and room for the initials of the witnessing pharmacist or other regulated health professional. A sample is below.
Sample PA witness log
|
Client name:
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
Date & time dispensed
|
Rx or transaction number
|
Quantity witnessed
|
Quantity unwitnessed ("carries")
|
Total quantity dispensed
|
Address of delivery
|
Time of delivery
|
Pharmacist / other health professional initials
|
Client sig
|
PharmaCare PA witness fee claim flowchart
Reference the PharmaCare PA witness fee claim flowchart (PDF, 82KB).
Resources
December 24 update: Nicotine buccal pouches no longer Schedule II drugs in B.C., but still subject to Health Canada rules
As of December 15, 2025, buccal pouches are no longer designated as Schedule II drugs in B.C.; however, they remain subject to the rules of the Health Canada Supplementary Rules Respecting Nicotine Replacement Therapies Order (PDF, 168KB).
These scheduling changes mean that the following nicotine replacement therapy products must remain behind the counter and require a client to consult with a pharmacist to access:
- Tobacco-free nicotine buccal pouches
The following products are no longer required to be sold behind the pharmacy counter. They must contain 4 mg or less of nicotine.
- Nicotine sprays or inhalers
The scheduling change does not impact nicotine gum, lozenges or transdermal nicotine patches, as they were never required to be sold behind the pharmacy counter.
Background
As described in the February 2024 PharmaCare Newsletter (PDF, 652KB), the Drug Schedules Regulation was amended to designate buccal nicotine pouches as Schedule II drugs; this meant that they must be sold by pharmacies only, for therapeutic use, and kept behind the counter. The regulation change was made to prevent recreational use of the products, especially by youth.
Following the provincial regulation, Health Canada enacted the Health Canada Supplementary Rules Respecting Nicotine Replacement Therapies Order (PDF, 168KB) on August 15, 2024. The Order applies to the sale of buccal nicotine pouches classified as natural health products that deliver 4 mg of nicotine or less per dose and are sold as a nicotine replacement therapy to help adults quit smoking, and not for recreational use or for non-smokers.
The Order requires the pouches to be sold exclusively by a pharmacist or an individual supervised by a pharmacist and kept behind the pharmacy counter.
The Order also restricts flavours to mint and menthol (to reduce appeal to youth), prohibits packaging that appeals to youth, requires a prominent warning about nicotine addiction on the package, and prohibits advertising that suggests a purpose other than smoking cessation (i.e., recreational).
The federal Order has allowed the BC College of Pharmacists to repeal the 2024 provincial amendment to the Drug Schedules Regulation.
Supporting clients to quit nicotine
Pharmacists are always encouraged to sign people up for the BC Smoking Cessation Program, which covers the full cost of non-prescription nicotine replacement therapy (NRT) (e.g., gum, lozenges, patches) and some or all the cost of prescribed medications. Learn more at Smoking Cessation Program – information for health professionals.
As part of providing the Minor Ailments and Contraception Service (MACS), B.C. pharmacists can assess and prescribe for nicotine dependence. Learn more at Minor Ailments and Contraception Service (MACS).
Resources
- Health Canada Supplementary Rules Respecting Nicotine Replacement Therapies Order (PDF, 168KB)
- Drug Schedules Regulation
- February 2024 PharmaCare Newsletter (PDF, 652KB)
- Smoking Cessation Program – information for health professionals
- Minor Ailments and Contraception Service (MACS)

About the PharmaCare Newsletter
The PharmaCare Newsletter is published on the first Wednesday of each month, with occasional mid-month releases. The PharmaCare Newsletter communicates drug listings, PharmaCare policy, PharmaNet procedures, and other pertinent information for PharmaCare providers and health care partners.
Information in previous newsletters is accurate as of the date it was published. Newsletters are not retroactively updated when policy, procedures or other information changes. Refer to the most recent mention of a topic for up-to-date information.
Search past newsletters on the Newsletter search page.
Subscribe
Enter your email address to subscribe to updates of this page.
 The PharmaCare Newsletter team works from the territory of the Lekwungen People, including the Songhees and Esquimalt Nations. Our gratitude extends to them, and all the Indigenous Peoples on whose territories and lands we build relationships.
The PharmaCare Newsletter team works from the territory of the Lekwungen People, including the Songhees and Esquimalt Nations. Our gratitude extends to them, and all the Indigenous Peoples on whose territories and lands we build relationships.
BC PharmaCare counts on pharmacy and device providers to practise cultural safety and humility.
To learn more, read Coming Together for Wellness, a series of articles by First Nations Health Authority (FNHA) and PharmaCare, and consider taking the online San’yas Indigenous Cultural Safety course.
Active advisories
spironolactone tablets; disopyramide capsules; olanzapine for injection; peginterferon alfa-2a (Pegasys®) injection
Visit Drug shortages for full list and details.




