Colorectal Cancer Part 2
Part 2: Follow-up of Colorectal Cancer and Precancerous Lesions (Polyps)
Effective Date: April 13, 2022
Recommendations and Topics
- Scope
- Key Recommendations
- Epidemiology
- Surveillance Following prior Colonoscopy with or without Precancerous Lesions
- Follow-up after CRC with Curative Resection
- Resources
Scope
This guideline provides follow-up recommendations for patients after curative resection of colorectal cancer (CRC) or colorectal precancerous lesions (polyps) to prevent the development of subsequent CRC. It does not apply to patients with colonic hereditary predisposition syndromes or inflammatory bowel disease. Recommendations for these patients and for the detection of CRC or precancerous lesions in asymptomatic patients are found in BCGuidelines.ca: Screening for the Purposes of Colorectal Cancer Prevention and Detection in Asymptomatic Adults.
Key Recommendations
- Removal of colorectal precancerous lesions (polyps) can prevent CRC.
- Early detection and treatment of recurrent CRC may prolong patient survival.
- Individuals with a history of precancerous lesions or CRC are at an increased risk for CRC in the future.
- Colonoscopy is the standard of care follow-up procedure to detect CRC or precancerous lesions.
- Individuals undergoing colonoscopy with removal of precancerous lesions should have a surveillance colonoscopy:
- In 10 years following removal of 1 to 4 low risk precancerous lesions,1
- In 3 years following removal of 5 or more low risk precancerous lesions,
- In 3 years following complete removal of a high risk precancerous lesion,
- Within 6 months if a large precancerous lesion is removed in a piecemeal fashion.
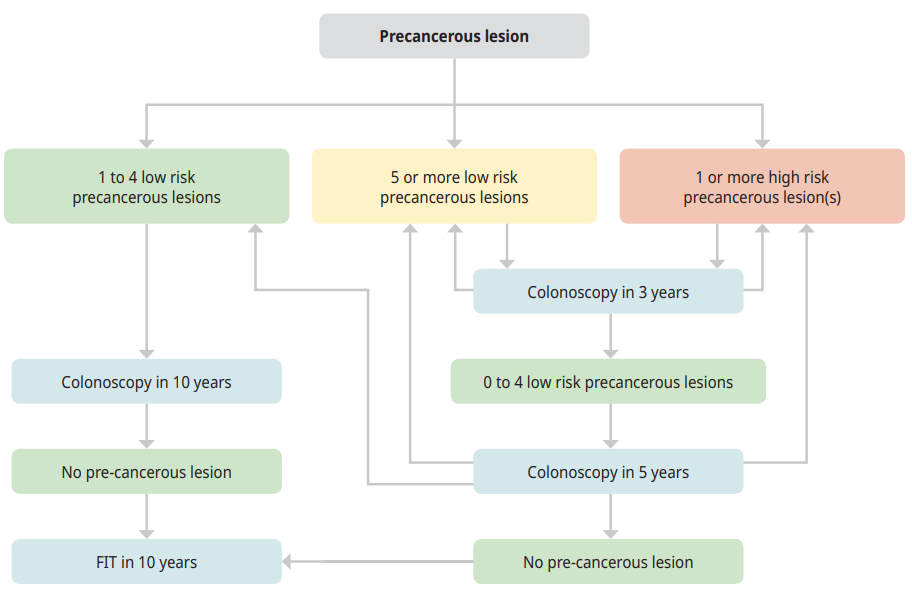
- The findings of the first surveillance colonoscopy will determine the timing of further colonoscopies or whether the individual returns to screening with fecal immunochemical test (FIT) (see Figure 1: Algorithm for surveillance colonoscopy).
- Patients followed by colonoscopy do not require FIT.
Epidemiology
Detection and removal of precancerous lesions (polyps) has been clearly demonstrated to reduce CRC incidence and mortality, and identification of CRC at an early stage markedly increases patient survival. Individuals who have had a high risk precancerous lesion resected are at an increased risk of future high risk precancerous lesions and CRC; colonoscopy surveillance at regular intervals is recommended (see below).2–4
The risk of a precancerous lesion becoming malignant is greatest for ‘high risk’ lesions (also known as advanced adenomas), which are defined as having any of the following:
- adenomas with villous features
- adenomas with high grade dysplasia
- adenomas or sessile serrated lesion (SSL) ≥ 10 mm (as measured by the colonoscopist at the time of excision)
- sessile serrated lesions (SSLs) with cytologic dysplasia
- Traditional serrated adenomas (TSAs)
- hyperplastic polyps ≥ 10 mm (as measured by the colonoscopist at the time of excision).
Precancerous lesions that do not meet the above criteria are classified as low risk precancerous lesions.
For individuals who have undergone curative resection of CRC, the majority of recurrences will become apparent in the first 2 to 3 years after the primary tumour resection.5
Management
Surveillance Following Prior Colonoscopy With or Without Precancerous Lesions
If a patient has been managed through the British Columbia (BC) Colon Screening Program (CSP), it is the intention that they will be contacted, and follow-up arranged at the appropriate interval. It is always helpful if patients can be advised of the expected interval so they can monitor their own healthcare needs.
Following removal of precancerous lesions
Hyperplastic polyps (HPs) < 10mm have no potential for malignant transformation and following removal require no special surveillance.
Most of CRCs arise from precancerous lesions. Two major types of precancerous lesions are found in the colon and rectum: adenomas and serrated lesions. Amongst the serrated lesions, sessile serrated lesions (SSLs) and traditional serrated adenomas (TSAs) are considered to have the potential for malignant transformation. HPs > 10mm have some potential for malignant transformation and are considered high risk lesions.
Individuals having multiple precancerous lesions are also at higher risk of CRC. This was previously defined as 3 or more precancerous lesions; however, based on recent evidence, the risk of CRC appears to increase once 5 or more precancerous lesions are detected.2,6 If the number of precancerous lesions removed during an individual’s lifetime is 10 or more, then referral to the Hereditary Cancer Program for evaluation of a potential genetic predisposition to CRC is recommended.7
The importance of a high-quality baseline colonoscopy cannot be overstated. A complete exam to the cecum, an adequate bowel preparation, and careful inspection of the mucosa with optimal polypectomy technique are associated with a decreased risk of CRC and CRC death.8–11 The BC CSP monitors these colonoscopy quality indicators and provides feedback to individual physicians, endoscopy units and health authorities regarding performance relative to the provincial average and accepted benchmarks. In addition, CSP supports quality improvement initiatives.
Table 1: Surveillance Recommendations1,12
| Findings at first colonoscopy | Recommendations for first follow-up surveillance colonoscopy | Recommendations for subsequent surveillance colonoscopy |
|---|---|---|
|
Patients with no polyps or only hyperplastic polyps < 10 mm* |
No surveillance required. Resume screening as per: BCGuidelines.ca: Screening for the Purposes of Colorectal Cancer Prevention and Detection in Asymptomatic Adults | Not applicable |
| Patients with 1 to 4 low risk precancerous lesions (< 10 mm, tubular adenomas with only low-grade dysplasia or SSLs without dysplasia) |
Follow-up colonoscopy in 10 years |
As per findings at each surveillance colonoscopy |
| Patients with 5 or more low risk precancerous lesions (< 10 mm, tubular adenomas with only low-grade dysplasia or SSLs without dysplasia) | Follow-up colonoscopy in 3 years | If 0 to 4 low risk lesions identified, then follow-up colonoscopy at 5 years and then as per colonoscopy findings |
| Patients with 1 or more high risk lesion(s) | Follow-up colonoscopy in 3 years | If 0 to 4 low risk lesions identified, then follow-up colonoscopy at 5 years and then as per colonoscopy findings |
Individuals with large precancerous lesions removed in a piecemeal fashion (rather than in a single piece) are at risk of residual precancerous tissue. Repeat colonoscopy to assess the site of lesion removal is recommended at 6 months. Further surveillance colonoscopy intervals will be based on several factors and will be at the discretion of the physician performing the colonoscopy.
Figure 1: Algorithm for surveillance colonoscopy1,12,13
Controversies in Care
Recommendations for colonoscopy surveillance following removal of precancerous lesions (polyps) are evolving. There is emerging data that an individual with 1 or 2 low risk adenomas has a reduced risk of CRC compared to the general population14,15 and a similar risk of high risk precancerous lesions on surveillance colonoscopy as those individuals who had a normal baseline colonoscopy.1,16,17 Similarly, individuals with 3 to 4 low risk adenomas do not appear to have an increased CRC incidence or mortality when compared to those with a no precancerous lesions or 1 to 2 low risk adenomas at baseline colonoscopy.2,6 In addition, there is evidence to support that individuals with 1 to 4 low risk SSLs have similar outcomes to those with 1 to 4 low risk adenomas.3 The British13 and European1 guidelines do not recommend surveillance colonoscopy following the removal of 1 to 4 low risk precancerous lesions but recommend these individuals return to average risk screening (i.e., resume FIT every 2 years) within organized screening programs. The United States Multi-Society Task Force guidelines18 continue to advocate for colonoscopy surveillance but have increased the interval to 7 to 10 years for individuals with 1 or 2 low risk precancerous lesions and 3 to 5 years following polypectomy of 3 or 4 low risk precancerous lesions. In Canada, Ontario has recommended that individuals with 1 or 2 low risk adenomas have a FIT in 5 years rather than a surveillance colonoscopy.17 It is anticipated that other Canadian provinces will also decrease the use of surveillance colonoscopy in these lower risk individuals by either increasing the colonoscopy interval or having them return to screening with FIT. The literature and the BC experience through BC Colon Screening Program outcomes are being closely monitored on a continuous basis and these guidelines may be revised in future if indicated.
Follow-up after CRC with curative resection
The goal of follow-up after resection is to identify recurrent disease or metastases and to detect subsequent precancerous lesions. These recommendations are generally expert consensus-based, reflecting national and international standards. Individuals with specific genetic syndromes should follow specialist recommendations. Patients with significant co-morbidities, very advanced age or limited 5-year life expectancy are not routinely offered surveillance.
Follow-up visits
Diligent follow-up after a cancer diagnosis is extremely important. Factors such as patient and practitioner mobility, “orphaned” patients, multi-practitioner care, and long intervals between follow-up visits may all predispose to missed follow-up. Careful use of Electronic Medical Records (EMRs) reminders is recommended. Be sure clarity is established regarding the most responsible practitioner (i.e., surgeon, oncologist, or family physician). It is important for the patient and practitioner(s) to understand who is taking responsibility for coordinating this follow-up. Engaging the patient in their anticipated follow-up is important.
Over the first 5 years, follow-up will consist of various examinations and intervals, including:
- History and physical
- Labs and tumour markers
- Diagnostic imaging
- Colonoscopy
The guidance below is excerpted from BC Cancer’s Follow-up and Surveillance of Colon Cancer Patients Treated with Curative Intent, please refer to this document for detailed information.19
Stage 0-I:
- Repeat colonoscopy is recommended in 1 year (6 months if complete colonoscopy was not performed at time of initial diagnosis), and if normal, in 3 years, and if normal every 5 years thereafter.
- There is no evidence of improved survival with routine imaging or blood work.
- Performance of FIT is unnecessary in patients undergoing colonoscopy surveillance.20
Stage II-III:
Follow up visits should be conducted:
- Every 3-6 months for the first 3 years
- Every 6 months for the next 2 years for a total of 5 years of surveillance
Each follow-up visit should include:
- Focused history and physical examination 21, 22
- Monitor for gastrointestinal and constitutional symptoms, including unexplained weight loss.
- Perform rectal exam at least annually.
- Carcinoembryonic antigen (CEA) tumour marker level should be checked at each follow-up visit. If CEA is elevated, repeat test within 28 days. Significantly elevated levels, usually at least doubling, can indicate hepatic or pulmonary metastases, and therefore a CT scan of chest and abdomen should be performed. If CEA continues to rise consultation should be obtained.
- Routine laboratory investigations, such as liver chemistry, in the absence of symptoms are generally not useful.
- FIT is unnecessary in patients undergoing colonoscopy surveillance.20
Chest, abdominal and pelvic imaging (CT scan preferable) should be done a minimum of 2 times over the first 3 years of follow-up (suggested at 12 months and 36 months). For those with advanced stage cancers or undergoing chemotherapy, follow the recommendations of the oncologist.22, 23 A chest CT scan is recommended for every 12 months for the first 3 years in cases of advanced cancer or rectal cancer.22, 24 Routine CT scanning is not recommended beyond 5 years. There is little evidence to show a survival benefit for routine chest x-ray for post CRC resection patients.25 Magnetic resonance imaging (MRIs) are generally reserved for the initial evaluation of rectal cancers.
Repeat colonoscopy is recommended in 1 year (6 months if complete colonoscopy was not performed at time of initial diagnosis), and if normal, in 3 years, and if normal every 5 years thereafter until age 75, then individualized based on the health and wishes of the patient.22, 26
Any test abnormalities, changes or new signs and symptoms should warrant investigation and referral to the primary oncologist.
Stage IV treated with curative-intent metastatectomy (Stage IV NED)
- No standard guidelines currently exist for surveillance in Stage IV NED and are as determined by the treating oncologist.
- May follow the recommendations as per Stage II-III above.
Resources
References
- Hassan C, Antonelli G, Dumonceau J-M, Regula J, Bretthauer M, Chaussade S, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2020. Endoscopy. 2020 Aug;52(08):687–700.
- Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. JAMA. 2018 May 15;319(19):2021.
- He X, Hang D, Wu K, Nayor J, Drew DA, Giovannucci EL, et al. Long-term Risk of Colorectal Cancer After Removal of Conventional Adenomas and Serrated Polyps. Gastroenterology. 2020 Mar;158(4):852-861.e4.
- Cross AJ, Robbins EC, Pack K, Stenson I, Kirby PL, Patel B, et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut. 2020 Sep;69(9):1645–58.
- Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Colorectal Group, editor. Cochrane Database Syst Rev [Internet]. 2019 Sep 4 [cited 2021 Oct 15];2019(9). Available from: http://doi.wiley.com/10.1002/14651858.CD002200.pub4
- Wieszczy P, Kaminski MF, Franczyk R, Loberg M, Kobiela J, Rupinska M, et al. Colorectal Cancer Incidence and Mortality After Removal of Adenomas During Screening Colonoscopies. Gastroenterology. 2020 Mar;158(4):875-883.e5.
- Davila RE, Rajan E, Baron TH. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006 Apr;63(4):546–57.
- Atkin W, Wooldrage K, Sasieni P, Duffy SW, Cross AJ. Colorectal adenomas, surveillance, and cancer – Authors’ reply. Lancet Oncol. 2017 Aug;18(8):e428.
- Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N Engl J Med. 2014 Apr 3;370(14):1298–306.
- Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality Indicators for Colonoscopy and the Risk of Interval Cancer. N Engl J Med. 2010 May 13;362(19):1795–803.
- Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, et al. Incomplete Polyp Resection During Colonoscopy—Results of the Complete Adenoma Resection (CARE) Study. Gastroenterology. 2013 Jan;144(1):74-80.e1.
- Dubé C, McCurdy BR, Bronstein, T, Pollett A, Baxter NN, Morgan D, et al. ColonCancerCheck Recommendations for Post-Polypectomy Surveillance [Internet]. Cancer Care Ontario; 2019 [cited 2021 Oct 15]. Available from: https://www.cancercareontario.ca/en/content/coloncancercheck-recommendations-post-polypectomy-surveillance
- Rutter MD, East J, Rees CJ, Cripps N, Docherty J, Dolwani S, et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020 Feb;69(2):201–23.
- Cottet V, Jooste V, Fournel I, Bouvier A-M, Faivre J, Bonithon-Kopp C. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012 Aug;61(8):1180–6.
- Løberg M, Kalager M, Holme Ø, Hoff G, Adami H-O, Bretthauer M. Long-Term Colorectal-Cancer Mortality after Adenoma Removal. N Engl J Med. 2014 Aug 28;371(9):799–807.
- Lee JK, Jensen CD, Levin TR, Doubeni CA, Zauber AG, Chubak J, et al. Long-term Risk of Colorectal Cancer and Related Death After Adenoma Removal in a Large, Community-based Population. Gastroenterology. 2020 Mar;158(4):884-894.e5.
- Dubé C, Yakubu M, McCurdy BR, Lischka A, Koné A, Walker MJ, et al. Risk of Advanced Adenoma, Colorectal Cancer, and Colorectal Cancer Mortality in People With Low-Risk Adenomas at Baseline Colonoscopy: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017 Dec;112(12):1790–801.
- Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, et al. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020 Mar;91(3):463-485.e5.
- BC Cancer. Follow-up and Surveillance of Colon Cancer Patients Treated with Curative Intent [Internet]. BC Cancer; 2018 [cited 2021 Nov 3]. Available from: http://www.bccancer.bc.ca/books/colon/follow-up-and-surveillance-of-colon-cancer-patients-treated-with-curative-intent
- Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, et al. Guidelines for Colonoscopy Surveillance after Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society,. CA Cancer J Clin. 2006 May 1;56(3):143–59.
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen Y-J, Ciombor KK, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Mar;19(3):329–59.
- Desch CE, Benson AB, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al. Colorectal Cancer Surveillance: 2005 Update of an American Society of Clinical Oncology Practice Guideline. J Clin Oncol. 2005 Nov 20;23(33):8512–9.
- Pfister DG, Benson AB, Somerfield MR. Surveillance Strategies after Curative Treatment of Colorectal Cancer. N Engl J Med. 2004 Jun 3;350(23):2375–82.
- NCCN Clinical Practice Guidelines in Oncology - Colon Cancer.
- Gan S. Surveillance of patients following surgery with curative intent for colorectal cancer. World J Gastroenterol. 2007;13(28):3816.
- Short M, Maj M, Maj B, Domagalski J. Colorectal Cancer Screening and Surveillance. Am Fam Physician. 2015 Jan 15;91(2):93–100.
Abbreviations
CEA
CRC
CT
EMRs
FIT
MRI
HP
SSL
TSA
Carcinoembryonic Antigen
Colorectal cancer
Computed tomography
Electronic Medical Records
Fecal immunochemical test
Magnetic resonance imaging
Hyperplastic polyps
Sessile serrated lesions
Traditional serrated adenomas
Practitioner resources
- BC Colon Screening Program: http://www.bccancer.bc.ca/screening/colon
- Hereditary Cancer Program at the BC Cancer Agency: www.bccancer.bc.ca
- Pathways: https://pathwaysbc.ca/login
- UBC CPD BC Cancer Primary Care Learning Sessions - Colorectal Cancer: https://elearning.ubccpd.ca/
- Canadian Cancer Society: www.cancer.ca
- Colon Cancer Canada: www.coloncancercanada.ca
- Public Health Agency of Canada: Cancer
Patient, Family and Caregiver resources
- BC Colon Screening Program: http://www.bccancer.bc.ca/screening/colon
- BC Colon Screening: Facts and Myths
- BC Cancer: Hereditary Cancer Program
- HealthlinkBC: Health information, translation services, Health Service Navigators and dieticians, www.healthlinkbc.ca or by telephone at 811.
- Canadian Cancer Society: www.cancer.ca
- Colon Cancer Canada: www.coloncancercanada.ca
- Public Health Agency of Canada: Cancer
Associated Documents
The following documents accompany this guideline:
- BCGuidelines.ca: Screening for the Purposes of Colorectal Cancer Prevention and Detection in Asymptomatic Adults
- BC Cancer: Colon Screening Program: Colonoscopy Referral Form
- BC Cancer: Standard Out-Patient Laboratory Requisition Form
- Associated Document - List of Contributors (PDF, 61KB)
This guideline is based on scientific evidence current as of the effective date.
This guideline was developed by the Guidelines and Protocols Advisory Committee in collaboration with the Provincial Laboratory Medicine Services, and adopted under the Medical Services Act and the Laboratory Services Act.
For more information about how BC Guidelines are developed, refer to the GPAC Handbook available at
BCGuidelines.ca: GPAC Handbook.
THE GUIDELINES AND PROTOCOLS ADVISORY COMMITTEE
The principles of the Guidelines and Protocols Advisory Committee are to:
- encourage appropriate responses to common medical situations
- recommend actions that are sufficient and efficient, neither excessive nor deficient
- permit exceptions when justified by clinical circumstances
Contact Information:
Guidelines and Protocols Advisory Committee
PO Box 9642 STN PROV GOVT
Victoria, BC V8W 9P1
Email: hlth.guidelines@gov.bc.ca
Website: www.BCGuidelines.ca
Disclaimer
The Clinical Practice Guidelines (the guidelines) have been developed by the guidelines and Protocols Advisory Committee on behalf of the Medical Services Commission. The guidelines are intended to give an understanding of a clinical problem, and outline one or more preferred approaches to the investigation and management of the problem. The guidelines are not intended as a substitute for the advice or professional judgment of a health care professional, nor are they intended to be the only approach to the management of clinical problem. We cannot respond to patients or patient advocates requesting advice on issues related to medical conditions. If you need medical advice, please contact a health care professional.


 TOP
TOP