Cardiovascular Disease - Primary Prevention
Effective Date: December 8, 2021
Recommendations and Topics
- Scope
- Key Recommendations
- Assessment of Risk
- Management of Risk
- When to Refer Patients to a Specialist
- Controversies in Care
- Management of Other Clinical Conditions
- Methodology
- Resources
Scope
This guideline provides recommendations on the primary prevention of atherosclerotic cardiovascular disease (ASCVD/CVD) in adults aged ≥19 years without clinical CVD. It does not apply to patients with a known history of CVD or who currently have signs or symptoms of CVD, as this would require treatment and secondary prevention. The recommendations include how to assess a patient’s risk of CVD and how to manage their CVD risk factors.
Familial hypercholesterolemia (FH) and other genetic dyslipidaemias are out of scope of this guideline. Practitioners are recommended to access Canadian Cardiovascular Society guidelines that address this condition.1 For updated guidance on secondary prevention practitioners are recommended to access the 2021 Canadian Cardiovascular Society guidelines.2
Key Recommendations
- Assess CVD risk in all asymptomatic adults ≥40 years of age [Strong Recommendation, Strong Evidence].2–5
- Health behaviour change (e.g., smoking cessation, healthy diet) is recommended as the first-line intervention for all risk groups in CVD primary prevention. Pharmacological management is recommended for high risk groups [Strong Recommendation, Strong Evidence].2,4,5
- Initiate statin therapy only after objectively evaluating the person’s individual risks, benefits and preferences, and by having an individualized discussion with the patient. Initiate pharmaceutical management after considering the patient’s overall individual risk. Treatment with a statin is expected to result in a significant reduction (30 - 50%) in the elevated baseline lipid levels [Strong Recommendation, Strong Evidence]. 5–8
- Reducing LDL-C using statin and/or non-pharmacological management is recommended as each 1 mmol/L decrease in LDL-C results in a 20-22% relative risk reduction of major vascular events [Strong Recommendation, Strong Evidence].2,9
- The use of aspirin to reduce risk of morbidity or mortality may only be beneficial to certain individuals. [Strong Recommendation, Strong Evidence]. 5,10,11
- Recommendation against the use of over-the-counter omega-3 PUFA to reduce CVD risk. [Strong Recommendation, Strong Evidence].
Assessment of Risk
Who to assess
Consider assessing CVD risk in:
- all asymptomatic men and women ≥40 years to establish a baseline4,5;
- all patients with pre-existing risk-related conditions (e.g., HTN, DM, CKD); and
- all patients with a known family history of premature CVD (defined as men aged <55 years and women aged <65 years in first degree relatives).*
A patient may be reassessed in 1 to 5 years depending on their initial risk assessment or if their risk factors change significantly. For further details, refer to Appendix A: Primary Prevention of Cardiovascular Disease Algorithm (PDF, 168KB)
Risk Assessment Investigations
-
Risk assessment tool: The Framingham Risk Score (FRS) is recommended.†
The FRS, or any CVD risk assessment tool, is a risk estimation only of a patient’s CVD risk. Since these scores are plus or minus several percentage points, it is important to consider modifying the risk estimation based on other known risk factors (e.g., family history, ethnicity) and a practitioner’s clinical judgement. For example, the Canadian Cardiovascular Society (CCS) suggests that among individuals 30 - 59 years of age without diabetes, the presence of a positive history of premature CVD in first degree relatives increases a patient’s FRS by approximately 2-fold.4
- In addition to the FRS, other risk assessment tools include Absolute CVD Risk/Benefit Calculator from James McCormack (for patients ≤80 years), the University of Edinburgh Cardiovascular Risk Calculator, the United Kingdom Prospective Diabetes Study (UKPDS) risk calculator that estimates the 10-year CHD and stroke risk for adults with type 2 diabetes and QRISK3 risk calculator (for patients ≤84 years). For additional details on the risk assessment tools, refer to Associated Document: Resource Guide for Physicians - Tools for Primary Prevention of Cardiovascular Disease. Paper-based scores use groups of measurements for the risk factors to assign points; and online calculators use the exact measurements for the risk factors. A risk score from an online calculator allows for a more individualized estimate of risk .
- Non-modifiable risk factors include4–6:
- age - chronological and biological age,
- biological sex (men)
- family history of CVD or familial hyperlipidemia (1st degree relative with ASCVD - men <55 years and women <65 years)
- ethnicity (First Nations,12 South Asians (defined as Indian, Pakistani, Bangladeshi or Sri Lankan origin))13 For any individual, it is imperative that the health needs of that individual as it relates to their racial/ethnic background (e.g., South Asians) is critically examined to ensure culturally appropriate medical and health decisions.14
- chronic kidney disease, chronic inflammatory diseases (e.g., rheumatoid arthritis, psoriatic arthritis, systemic lupus erythematosus, vasculitis (polyarteritis nodosa)), HIV infection, hypertensive diseases of pregnancy, Polycystic Ovarian Syndrome, gestational diabetes.
* First degree relatives have a blood relationship to the patient: parents, brothers, sisters and children.
† Though the FRS is recommended, there are other risk assessment tools. Refer to Associated Document: Resource Guide for Physicians – Tools for Primary Prevention of Cardiovascular Disease (PDF, 109KB).
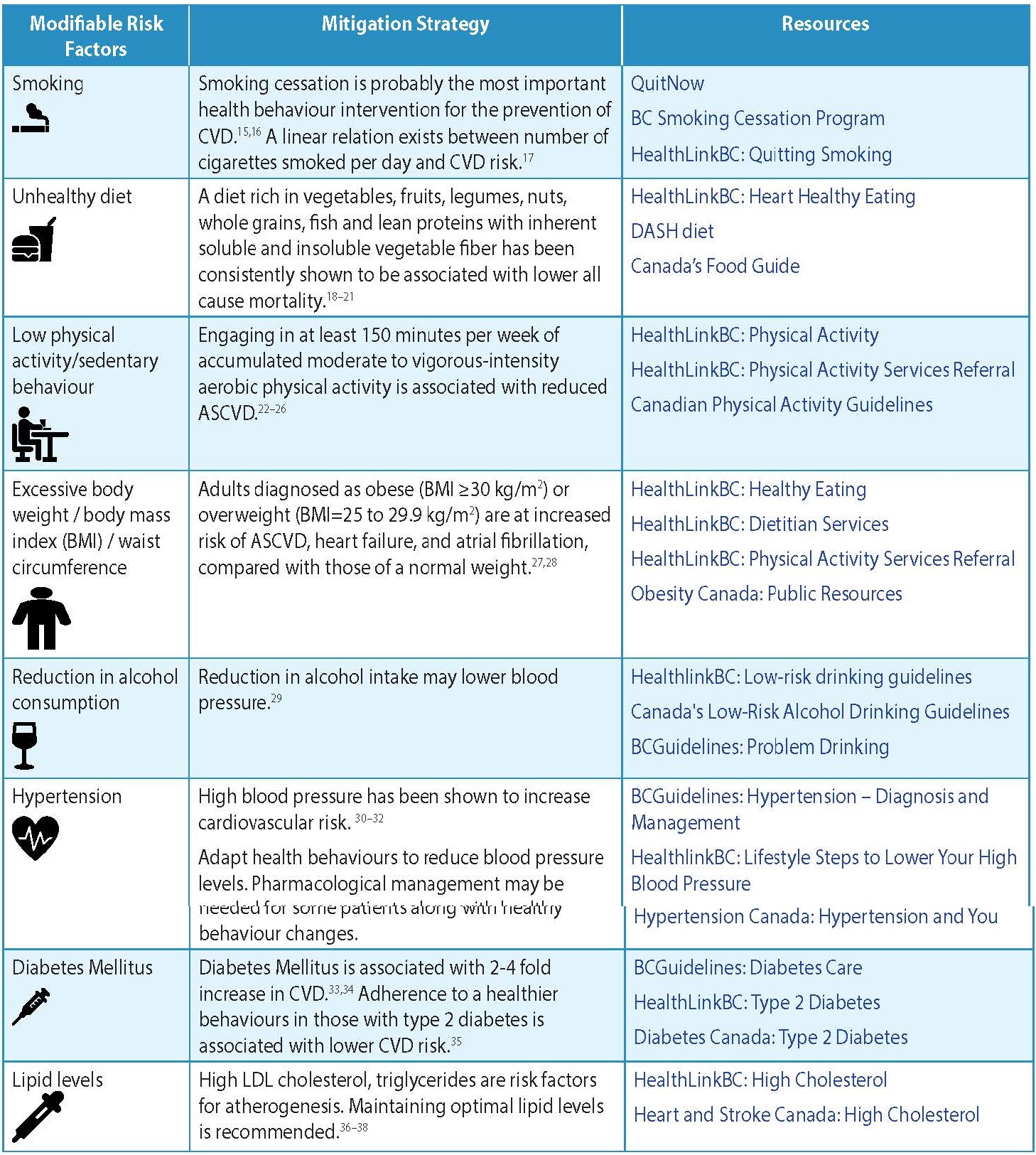
- The modifiable risk factors are listed in Table 1: Modifiable Risk Factors for CVD.
Table 1: Modifiable Risk Factors for CVD4-6
- Physical examination - Besides regular physical exam elements, conduct a focused cardiovascular physical examination, including assessing for any physical signs of dyslipidemias (premature corneal arcus, tendon xanthomas, and xanthelasmas).
- Test for lipids - order full lipid profile including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), non-high-density lipoprotein cholesterol§ (non-HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG).Non-fasting lipid testing is recommended.45
- Test for type 2 diabetes - order fasting plasma glucose (FPG) OR hemoglobin A1c level.
§ As of October 2013, a non-HDL-C measurement has been included in the full lipid profile or as a separate test on the Standard Outpatient Laboratory Requisition. For more information, refer to Appendix C: Lipid Testing in Primary Prevention of Cardiovascular Disease (PDF, 141KB).
- Assess Renal Function: eGFR test, urine ACR test
No other investigations are usually indicated in the risk assessment for asymptomatic patients unless stratification of intermediate risk patients is warranted (as outlined below). Although it may have a role in intermediate risk patients, for most patients, the routine testing of high-sensitivity C-reactive protein (hsCRP) is not indicated.2
- Lipoprotein(a) in Primary Prevention Screening
The most recent CCS guideline (2021) recommends measuring Lipoprotein(a) [Lp(a)] level once in a person’s lifetime as a part of the initial lipid screening. Earlier and more intensive behaviour modification and management of other ASCVD risk factors are recommended for those with a Lp(a) ≥50 mg/dL (or ≥100 nmol/L). The recommendation was based on recent studies suggesting the potential role of Lp(a) as a target of treatment in the future, although currently there is no evidence from randomized control trials that specifically lowering Lp(a) leads to reductions in CV outcomes. It should also be noted that commonly used lipid-lowering therapies (i.e. statins and ezetimibe) do not appreciably lower Lp(a). The only available lipid-lowering therapies that lead to substantial lowering of Lp(a) include PCSK9 inhibitors, niacin, and apheresis, but relatively limited evidence exists for their use in patients with high Lp(a). 2,46,47
- Assessment Stratification
The patient can be classified as low, intermediate, or high risk for CVD based on the risk assessment. Any patient that is considered very high risk or is symptomatic (defined as secondary prevention - out of the scope of this guideline) should be treated accordingly. The FRS defines low risk as <10%, intermediate risk as 10 - 19% and high risk as ≥20%. These groupings are an arbitrary convenience, not a scientifically validated stratification.
A patient in the intermediate risk group may warrant a secondary assessment to raise or lower their risk stratification. However, further investigations may not be appropriate if the results would not influence the decision of how to manage the risk or treat the patient.
Secondary assessment should be done on patients for whom treatment decisions are uncertain. These assessments may include carotid ultrasound, hsCRP, or coronary artery calcium (CAC) scoring. Updated guidance on the use of CAC is recommended by the CCS 2021 guidelines.2
Conduct a shared decision-making conversation regarding healthy behaviour modifications and if necessary pharmacological interventions. Consider using cardiovascular age during the discussion.
Cardiovascular (CV) age using the Cardiovascular Life Expectancy Model (CLEM) is calculated as the patient’s age minus the difference between his or her estimated remaining life expectancy (adjusted for coronary and stroke risk) and the average remaining life expectancy of Canadians of the same age and sex (chiprehab.com/index.html).4,48
Management of Risk
Healthy Behaviour
Healthy behaviour modifications need to be strongly advocated as the first-line intervention for all risk groups. Adequate explanations and support should be provided to patients, so they clearly understand the nature and significance of CVD, and that they have the primary responsibility for adopting the healthy behaviour changes required for reducing their risk.
Use the prevention visit code – 14066 for discussions related to management of modifiable risks. Diagnostics codes that require a prevention focused advice include smoking (786), unhealthy eating and medical obesity (783), physically inactive (785).
- Smoking: Promote smoking cessation and avoidance of second-hand smoke. Behavioral and pharmacotherapy interventions, alone or in combination, have been shown to improve rates of smoking cessation among the general adult population.49,50 Use a Screening, Brief Intervention and Referral to Treatment (SBIRT) approach.51 When talking to a patient about smoking: 1) Screen for use 2) Conduct a Brief Intervention by providing risks of behaviour 3) Assess for willingness to quit 4) Support behaviour change by connecting to resources or treatment.
- For support to quit, refer patients to:
- QuitNow at www.quitnow.ca/
- HealthLinkBC Quit Smoking – Patients can call 8-1-1 or visit the website www.healthlinkbc.ca/health-feature/quit-smoking.
- BC Smoking Cessation Program
- Smokers’ Helpline at 1-866-366-3667 or online at SmokersHelpline.ca
- For more information on effective pharmacological aids for smoking cessation, refer to BC Smoking Cessation program.
- Electronic cigarettes, also known as e-cigarettes, vaping (available with or without nicotine), may play a role as an aid in smoking cessation. At present time, their risk and benefits have not been clearly established51–54 and are not included as a pharmacological aid.
- Physical Activity: Support patient working towards 30 minutes or more of moderate to vigorous intensity physical activity on most days of the week (weekly total ≥150 minutes).25,55
Behavioural interventions for healthful diet and physical activity have been shown to generally improve participants’ dietary intake and physical activity levels at 6 to 12 months of followup.56 Techniques such as motivational interviewing techniques and brief action planning, that promote collaborative engagement with the patient, are more effective than exercise prescription alone for patients to achieve their physical activity goals.
- Exercise stress test may be warranted for previously sedentary people with additional risk factors for CVD who wish to undertake exercise more vigorous than brisk walking.4
- For patients who are sedentary, consider a graduated exercise program using Brief Action Planning (BAP).
- Engage the patients in completing a Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+) to help them determine their readiness. Refer them to an accessible exercise program (such as healthy heart programs).
- For assistance with personalized physical activity advice, refer patient to a physical activity expert at HealthLinkBC or by telephone at 8-1-1.
- Diet: Encourage a well-balanced diet. There are many dietary pathways to achieve CV risk reduction such as the Mediterranean diet (which emphasizes fruits, vegetables, legumes, whole grains and olive oil, with moderate consumption of fish, dairy products, poultry and minimizing meats and sweets) or the Dietary Approaches to Stop Hypertension (DASH) diet.4
- For assistance with personalized diet advice, refer patient to a dietitian at HealthLinkBC by telephone 8-1-1 or website: www.healthlinkbc.ca.
- Alcohol Consumption: Screen for alcohol abuse. Use a Screening, Brief Intervention, and Referral for Treatment (SBIRT) approach.
Follow-up to Healthy Behaviour Modifications
- Assess success of healthy behaviour intervention change at first follow-up.
- Assess cardiovascular risk using lipid profile (non-fasting)
- For those with elevated lipids from their initial risk assessment, they may be followed up with a lipid profile in 3 – 6 months. If elevated lipids are still a concern, consider pharmaceutical management.
- Studies consistently demonstrate a 20-22% relative risk reduction for each 1 mmol/L reduction in low-density lipoprotein cholesterol (LDL-C).9 The absolute risk reduction is thus dependent upon the baseline risk and the baseline LDL-C, as statin treatment will provide a greater absolute LDL-C lowering in those with higher baseline values.2
Table 2. Lipids levels that may be considered elevated relative to a patient’s risk level
| Risk Level (Patient's risk level as determined by risk assessment tools such as FRS) | LDL-C (mmol/L) considered elevated level | Non-HDL-C (mmol/L)considered elevated level |
ApoB (g/L) An ApoB test is not part of the initial risk assessment, but may be used as follow-up test |
|---|---|---|---|
| High | ≥ 2.0 | ≥ 2.6 | ≥ 0.8 |
| Intermediate | ≥ 3.5 | ≥ 4.2 | ≥ 1.05 |
| Low | ≥ 5.0 | ≥ 5.8 | ≥ 1.45 |
Both non-HDL-C and ApoB appear to be stronger predictors than LDL-C for major future cardiovascular events.2,57,58 Non-HDL-C may also be a better indicator of residual risk after statin therapy than LDL-C.59 ApoB is not available with lipid profiles unless diagnosis of complex dyslipidemia is indicated.
Pharmaceutical Management
Acetylsalicylic Acid (ASA) Therapy
ASA therapy in Primary Prevention: Evidence does not support the use of aspirin in low and intermediate risk patients. The evidence for use of aspirin in high risk patients is currently uncertain.5,60 Use of aspirin in people >75 may further heighten the risk of clinical significant bleeding.61
Statin Therapy
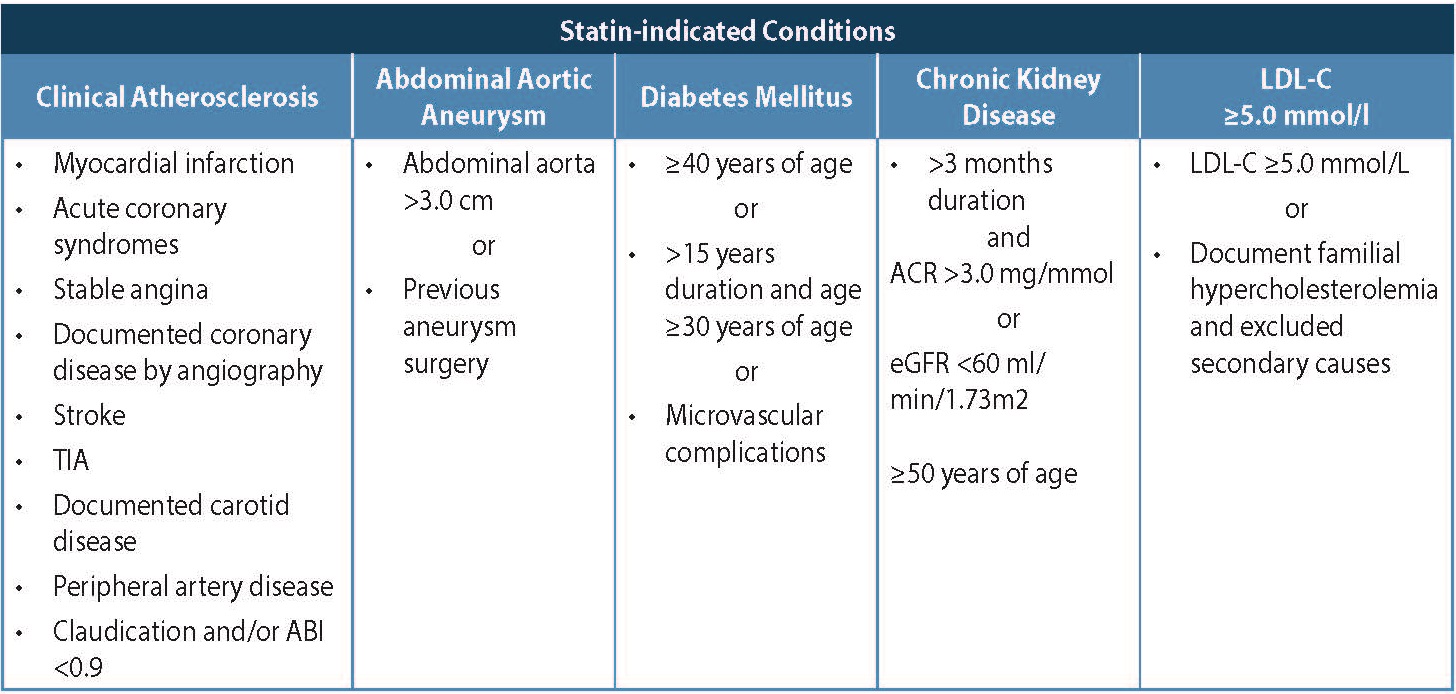
For those patients with DM, CKD, or Familial Hyperdyslipidemia, statin therapy is indicated along with healthy behaviour interventions. Table 3: Statin-Indicated Conditions below is adapted from CCS guidelines, 2016. 4
Table 3. Statin-Indicated Conditions4
A patient-specific discussion regarding the potential risks and benefits of statin use should be undertaken. A recent Cochrane review assessed the benefit of statin therapy and reported that all cause mortality was reduced by statins; as was combined fatal and non-fatal CVD, combined fatal and non-fatal CHD events and combined fatal and non-fatal stroke. The review reported adjusted Number Needed to Treat (NNT) for 5 years was 96 for all cause mortality and 56 for fatal and non-fatal chronic heart disease.7 The decision for initiating statin therapy should not be based on lipid levels alone and should be based on cardiovascular risk assessment.62
Two tools to assist in individualizing this discussion include:
- Absolute CVD Risk/Benefit Calculator, website: https://cvdcalculator.com/
- Cardiovascular Prevention Decision Aids, website: statindecisionaid.mayoclinic.org/
If statin therapy is decided upon, consider using a high potency statin (such as Atorvastatin or Rosuvastatin) considering efficacy and cost considerations. For dosages and adverse effects, refer to Appendix D: Pharmaceutical Table
Prior to the initiation of statin therapy:
- inform the patient of adverse effects63–65 - effects may include muscle pain/myopathy/weakness, rhabdomyolysis, cataracts, elevated blood glucose and diabetes, acute renal failure, and liver injury;
- educate the patient about any possible drug interactions with other prescribed medication, over-the-counter remedies and non-pharmaceuticals - consult a pharmacist or product monograph for a complete list; and
- emphasize the importance of long-term compliance - it is estimated that 75% of primary prevention patients aged >65 years old started on statins stop their therapy within 2 years.66
Follow-up to Statin Therapy
Within 3 - 6 months of the initiation of statin therapy, follow-up with the patient. This may include:
- Measure lipids with a non-HDL-C or an ApoB to assess patient adherence to statin therapy and any response to statin therapy (see Controversies in Care). A full lipid profile is not indicated. If both healthy behaviour intervention and a statin intervention have not been successful and lipids are still above target in the follow-up investigation, consider any other causes of elevated lipids (e.g., hypothyroidism, non-adherence).
- Inquire about any adverse effects. The risk of statin-induced serious muscle injury, including rhabdomyolysis, is <0.1%, and the risk of serious hepatotoxicity is ≈0.001%.65 If muscle pain or weakness is reported in patients, measure CK. In asymptomatic patients, CK is not necessary. CK elevation is of concern only when it is significantly elevated (i.e., >5X)63
- Measure liver transaminase enzyme (alanine aminotransferase (ALT)) only once within the first 3 months of starting statin. If a patient has elevated liver transaminase enzymes, (greater than 3X the normal) consider secondary causes.65
Further follow-ups as clinically needed. After the initial follow-up, routine monitoring of CK and ALT is not indicated for asymptomatic patients. More frequent routine monitoring with a full lipid profile, non-HDL-C or an ApoB is not considered necessary for the sole purpose of treat-to-target.
When to Refer Patients to a Specialist
Consider referral to a specialist when there is:
- Difficulty reaching treatment targets despite maximum-tolerated lipid-lowering therapy.
- Intolerance to or adverse effects of statin treatment. Statin intolerance needs to be well documented prior to referral.63
Controversies in Care
Statin Therapy in Primary Prevention
Both the Canadian Cardiovascular Society (CCS)4 and the American Heart Association (ACC/AHA)5 acknowledge that there is high interindividual variability in LDL-C levels attained with statin therapy. They agree that recent studies have demonstrated lower CVD event rates with moderate-intensity and high-intensity statin therapy that outweighed the observable risks.4,5,67 The 2016 USPSTF systematic review of statin therapy in primary prevention showed a reduced risk of all-cause and cardiovascular mortality and ASCVD events and noted greater absolute benefits in those at greater baseline risk.68 Both the CCS and ACC/AHA have recommended a more aggressive approach for statin use (see Table 4: Comparison of statin therapy recommendations between the CCS and ACC/AHA).
| Criteria | CCS Recommendations | ACC/AHA Recommendations |
|---|---|---|
| Risk Assessment tool | FRS - low risk, intermediate risk, high risk. |
Pooled Cohort Equations - elevated risk (≥7.5%), not elevated risk (≤7.5%). The 7.5% approximately equates to a FRS score of 15% +/- 3% (depending on the risk factors). |
| Intermediate risk | Treat with statins based on FRS (10 - 19%0 if LDL-C ≥3.5 mmol/L. |
Treat with statins based in Pooled Cohort Equations ASCVD risk ≥7.5% to <20% 10-year,
|
| High risk | Treat with statins based on FRS (≥20%). | Treat with statins based on Pooled Cohort Equations ≥7.5%. |
| LDL-C ≥5 mmol/L | Treat with statins. | Treat with statins. |
| DM |
Treat with statins for those age ≥40 years, >15-year duration for age ≥30 years (type 1 diabetes mellitus [DM]), or with the presence of microvascular disease. |
Treat with statins if patient aged 40 to 75 years. |
| CKD or high risk HTN | Treat with statins. | No specific recommendation. |
Statin Use for Primary Prevention in the Elderly Population
The CCS and the ACC/AHA guidelines both recommend discussion of statin use with elderly patients who are believed to be at higher risk.2,4,5,69 There are randomized controlled trials currently underway specifically assessing statins in primary prevention in this population.
Treatment Goals
- Both the CCS4 and the ACC/AHA5 acknowledge there is controversy regarding the use of lipid treatment targets. This guideline is aligned with the current CCS (2021) recommendations in that treatment with maximally tolerated statins is recommended. If thresholds are not achieved add on therapy should be considered.
A number of clinical conditions contribute significantly to the risk of developing CVD.
Blood Pressure Control
Support healthy behaviour modifications, followed by the use of antihypertensive medications when appropriate, with consideration for the presence of other CVD risk factors.
For more information, refer to BCGuidelines.ca - Hypertension: Diagnosis and Management.
Diabetes Care
Support healthy behaviour modifications followed by the use of medications when appropriate to control blood glucose. DM is a major risk factor for CVD, but a patient with DM does not need to be automatically considered high risk for CVD. CCS defines a patient with DM high risk for CVD with age ≥40 years, >15-year duration for age ≥30 years (type 1 diabetes mellitus), or with the presence of microvascular disease. While the current FRS now includes diabetic status to individualize a type 2 DM patient’s risk, use the United Kingdom Prospective Diabetes (UKPDS) risk calculator or table, website: www.dtu.ox.ac.uk/riskengine.
For more information, refer to BCGuidelines.ca - Diabetes Care.
CKD Management
In patients with CKD, the combination of simvastatin plus ezetimibe has shown benefit in reducing major atherosclerotic events when compared to placebo; however, no benefit on all-cause mortality has been demonstrated.70
For more information, refer to BCGuidelines.ca - Chronic Kidney Disease - Identification, Evaluation and Management of Adult Patients.
*BC Guidelines for these clinical conditions with effective dates before this one may not reflect the updates in this guideline (e.g., the addition of non-HDL-C as a measurement).
These guideline recommendations are tailored to support practice in British Columbia and are based on guidance by the Canadian Cardiovascular Society (CCS)2,4, American Cardiology/American Heart Association (ACC/AHA)5, European Society of Cardiology6. The guideline development working group used the AGREE II tool to assess the 6 domains and the overall guideline assessment. The working group looked at the three guidelines mentioned above carefully to identify the Scope of Purpose, Stakeholder Involvement, Rigor of Development, Clarity of Presentation, Applicability, Editorial Independence and made an assessment of the overall guideline quality. The AGREE II scores of the guidelines from the working group members showed some variation in domain scoring by individual members but overall agreement of the variation in the quality of these guidelines. The team gained a significant appreciation of both the methodology behind the three guidelines as well as the content and were able to use information from all of them in the GPAC guideline development. The working group started with the draft of the previous version of the GPAC guideline and studied the recommendations from the other three groups to inform this updated version. Where available, key references are provided. In situations where there is a lack of rigorous evidence, we provide best clinical opinion to support decision making and high-quality patient care. The guideline development process included significant engagement and consultation with primary care providers, specialists and key stakeholders, including the Provincial Laboratory Medicine Services. For more information about GPAC guideline development processes, refer to the GPAC handbook available at BCGuidelines.ca.
Resources
References
-
Brunham LR, Ruel I, Aljenedil S, Rivière J-B, Baass A, Tu JV, et al. Canadian Cardiovascular Society Position Statement on Familial Hypercholesterolemia: Update 2018. Canadian Journal of Cardiology. 2018 Dec;34(12):1553–63.
-
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Canadian Journal of Cardiology [Internet]. 2021 Mar 26 [cited 2021 Jun 29]; Available from: https://www.sciencedirect.com/science/article/pii/S0828282X21001653
-
Cooney Marie Therese, Dudina Alexandra, D’Agostino Ralph, Graham Ian M. Cardiovascular Risk-Estimation Systems in Primary Prevention. Circulation. 2010 Jul 20;122(3):300–10.
-
Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Canadian Journal of Cardiology. 2016 Nov;32(11):1263–82.
-
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation [Internet]. 2019 Mar 17 [cited 2020 Mar 12]; Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000678
-
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular riskThe Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020 Jan 1;41(1):111–88.
-
Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Smith GD, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews [Internet]. 2013 [cited 2020 Mar 12];(1). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004816.pub5/full/es
-
Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014 Aug 5;64(5):485–94.
-
Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA. 2016 Sep 27;316(12):1289.
-
Gelbenegger G, Postula M, Pecen L, Halvorsen S, Lesiak M, Schoergenhofer C, et al. Aspirin for primary prevention of cardiovascular disease: a meta-analysis with a particular focus on subgroups. BMC Medicine. 2019 Nov 4;17(1):198.
-
Zheng SL, Roddick AJ. Association of Aspirin Use for Primary Prevention With Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-analysis. JAMA. 2019 Jan 22;321(3):277–87.
-
Anand SS, Abonyi S, Arbour L, Balasubramanian K, Brook J, Castleden H, et al. Explaining the variability in cardiovascular risk factors among First Nations communities in Canada: a population-based study. The Lancet Planetary Health. 2019 Dec 1;3(12):e511–20.
-
Chiu M, Austin PC, Manuel DG, Tu JV. Comparison of cardiovascular risk profiles among ethnic groups using population health surveys between 1996 and 2007. CMAJ. 2010 May 18;182(8):E301–10.
-
Volgman AS, Palaniappan LS, Aggarwal NT, Gupta M, Khandelwal A, Krishnan AV, et al. Atherosclerotic Cardiovascular Disease in South Asians in the United States: Epidemiology, Risk Factors, and Treatments: A Scientific Statement From the American Heart Association. Circulation [Internet]. 2018 Jul 3 [cited 2020 Sep 17];138(1). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000580
-
Shields M, Wilkins K. Smoking, smoking cessation and heart disease risk: A 16-year follow-up study. Health Rep. 2013 Feb;24(2):12–22.
-
U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020.
-
Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004 Sep;364(9438):937–52.
-
Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017 Nov 4;390(10107):2050–62.
-
Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. New England Journal of Medicine. 2018 Jun 21;378(25):e34.
-
Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, et al. Association of Animal and Plant Protein Intake With All-Cause and Cause-Specific Mortality. JAMA Intern Med. 2016 Oct 1;176(10):1453–63.
-
Rees K, Takeda A, Martin N, Ellis L, Wijesekara D, Vepa A, et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2019 13;3:CD009825.
-
Ekelund U, Brown WJ, Steene-Johannessen J, Fagerland MW, Owen N, Powell KE, et al. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br J Sports Med. 2019 Jul;53(14):886–94.
-
Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016 14;5(9).
-
Kraus WE, Powell KE, Haskell WL, Janz KF, Campbell WW, Jakicic JM, et al. Physical Activity, All-Cause and Cardiovascular Mortality, and Cardiovascular Disease. Med Sci Sports Exerc. 2019;51(6):1270–81.
-
Tremblay MS, Warburton DER, Janssen I, Paterson DH, Latimer AE, Rhodes RE, et al. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011 Feb;36(1):36–46; 47–58.
-
Warburton DER, Bredin SSD. Reflections on Physical Activity and Health: What Should We Recommend? Canadian Journal of Cardiology. 2016 Apr 1;32(4):495–504.
-
Ma C, Avenell A, Bolland M, Hudson J, Stewart F, Robertson C, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017 Nov 14;359:j4849.
-
Czernichow S, Kengne A-P, Stamatakis E, Hamer M, Batty GD. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011 Sep;12(9):680–7.
-
Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OSM, Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health. 2017;2(2):e108–20.
-
Karmali Kunal N., Lloyd-Jones Donald M. Global Risk Assessment to Guide Blood Pressure Management in Cardiovascular Disease Prevention. Hypertension. 2017 Mar 1;69(3):e2–9.
-
Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016 Mar 5;387(10022):957–67.
-
Brunström M, Carlberg B. Association of Blood Pressure Lowering With Mortality and Cardiovascular Disease Across Blood Pressure Levels. JAMA Intern Med. 2018 Jan;178(1):28–36.
-
Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SRK, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010 Jun 26;375(9733):2215–22.
-
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASDThe Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2020 Jan 7;41(2):255–323.
-
Liu G, Li Y, Hu Y, Zong G, Li S, Rimm EB, et al. Influence of Lifestyle on Incident Cardiovascular Disease and Mortality in Patients with Diabetes Mellitus. J Am Coll Cardiol. 2018 Jun 26;71(25):2867–76.
-
Benjamin Emelia J., Virani Salim S., Callaway Clifton W., Chamberlain Alanna M., Chang Alexander R., Cheng Susan, et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation. 2018 Mar 20;137(12):e67–492.
-
Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. Journal of the American College of Cardiology. 2018 Sep 4;72(10):1141–56.
-
Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017 Aug 21;38(32):2459–72.
-
Schultz William M., Kelli Heval M., Lisko John C., Varghese Tina, Shen Jia, Sandesara Pratik, et al. Socioeconomic Status and Cardiovascular Outcomes. Circulation. 2018 May 15;137(20):2166–78.
-
Sumner Jennifer A., Khodneva Yulia, Muntner Paul, Redmond Nicole, Lewis Marquita W., Davidson Karina W., et al. Effects of Concurrent Depressive Symptoms and Perceived Stress on Cardiovascular Risk in Low‐ and High‐Income Participants: Findings From the Reasons for Geographical and Racial Differences in Stroke (REGARDS) Study. Journal of the American Heart Association. 5(10):e003930.
-
Steptoe A, Kivimäki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–54.
-
Havranek Edward P., Mujahid Mahasin S., Barr Donald A., Blair Irene V., Cohen Meryl S., Cruz-Flores Salvador, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease. Circulation. 2015 Sep 1;132(9):873–98.
-
Andermann A. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016 Dec 6;188(17–18):E474–83.
-
Herink M, Ito MK. Medication Induced Changes in Lipid and Lipoproteins. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 [cited 2020 Jun 5]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK326739/
-
Doran B, Guo Y, Xu J, Weintraub H, Mora S, Maron DJ, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and Nutrition Examination Survey III (NHANES-III). Circulation. 2014 Aug 12;130(7):546–53.
-
Saeed A, Kinoush S, Virani SS. Lipoprotein (a): Recent Updates on a Unique Lipoprotein. Curr Atheroscler Rep. 2021 Jun 19;23(8):41.
-
Langsted A, Nordestgaard BG. Genetics of Lipoprotein(a): Cardiovascular Disease and Future Therapy. Curr Atheroscler Rep. 2021 Jun 20;23(8):46.
-
Cooney MT, Dudina AL, Graham IM. Value and Limitations of Existing Scores for the Assessment of Cardiovascular Risk: A Review for Clinicians. Journal of the American College of Cardiology. 2009 Sep 29;54(14):1209–27.
-
Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the U.S. Preventive Services Task Force. Ann Intern Med. 2015 Oct 20;163(8):608–21.
-
Final Recommendation Statement: Tobacco Smoking Cessation in Adults, Including Pregnant Women: Behavioral and Pharmacotherapy Interventions | Document | United States Preventive Services Taskforce [Internet]. [cited 2020 Jun 1]. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions#citation1
-
Hargraves D, White C, Frederick R, Cinibulk M, Peters M, Young A, et al. Implementing SBIRT (Screening, Brief Intervention and Referral to Treatment) in primary care: lessons learned from a multi-practice evaluation portfolio. Public Health Reviews. 2017 Dec 29;38(1):31.
-
National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes [Internet]. Eaton DL, Kwan LY, Stratton K, editors. Washington (DC): National Academies Press (US); 2018 [cited 2020 Jun 1]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK507171/
-
Alzahrani T, Pena I, Temesgen N, Glantz SA. Association Between Electronic Cigarette Use and Myocardial Infarction. American Journal of Preventive Medicine. 2018 Oct 1;55(4):455–61.
-
Grana Rachel, Benowitz Neal, Glantz Stanton A. E-Cigarettes. Circulation. 2014 May 13;129(19):1972–86.
-
Warburton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. International Journal of Behavioral Nutrition and Physical Activity. 2010 May 11;7(1):39.
-
Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 [cited 2020 May 22]. (U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews). Available from: http://www.ncbi.nlm.nih.gov/books/NBK476368/
-
Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. The Lancet. 2019 Dec 14;394(10215):2173–83.
-
Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM - PubMed [Internet]. [cited 2021 Jul 2]. Available from: https://pubmed-ncbi-nlm-nih-gov.ezproxy.hlth.gov.bc.ca/31855562/
-
Sniderman Allan D., Williams Ken, Contois John H., Monroe Howard M., McQueen Matthew J., de Graaf Jacqueline, et al. A Meta-Analysis of Low-Density Lipoprotein Cholesterol, Non-High-Density Lipoprotein Cholesterol, and Apolipoprotein B as Markers of Cardiovascular Risk. Circulation: Cardiovascular Quality and Outcomes. 2011 May 1;4(3):337–45.
-
Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV. Bleeding Risks With Aspirin Use for Primary Prevention in Adults: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016 Jun 21;164(12):826.
-
Chiang KF, Shah SJ, Stafford RS. A Practical Approach to Low-Dose Aspirin for Primary Prevention. JAMA. 2019 Jul 23;322(4):301.
-
Byrne P, Cullinan J, Smith A, Smith SM. Statins for the primary prevention of cardiovascular disease: an overview of systematic reviews. BMJ Open. 2019 Apr 1;9(4):e023085.
-
Mancini GBJ, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, Prevention, and Management of Statin Adverse Effects and Intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol. 2016;32(7 Suppl):S35-65.
-
Toth PP, Patti AM, Giglio RV, Nikolic D, Castellino G, Rizzo M, et al. Management of Statin Intolerance in 2018: Still More Questions Than Answers. Am J Cardiovasc Drugs. 2018;18(3):157–73.
-
Newman Connie B., Preiss David, Tobert Jonathan A., Jacobson Terry A., Page Robert L., Goldstein Larry B., et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019 Feb 1;39(2):e38–81.
-
Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002 Jul 24;288(4):462–7.
-
Ridker PM, Mora S, Rose L. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016 May 1;37(17):1373–9.
-
Chou R, Dana T, Blazina I, Daeges M, Bougatsos C, Grusing S, et al. Statin Use for the Prevention of Cardiovascular Disease in Adults: A Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 [cited 2020 Jun 10]. (U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews). Available from: http://www.ncbi.nlm.nih.gov/books/NBK396415/
-
Gencer B, Marston NA, Im K, Cannon CP, Sever P, Keech A, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020 Nov 21;396(10263):1637–43.
-
Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. The Lancet. 2011 Jun 25;377(9784):2181–92
Abbreviations
ACC/AHA American Heart Association
ACR Albumin-to-Creatinine Ratio
AGREE II Appraisal of Guidelines for Research & Evaluation Instrument
ALT Alanine Aminotransferase
ApoB Apolipoprotein B
ASA Acetylsalicylic Acid
ASCVD/CVD Atherosclerotic cardiovascular disease
BAP Brief Action Planning
BMI Body Mass Index
CAC Coronary Artery Calcium
CCS Canadian Cardiovascular Society
CK Creatine Kinase
CKD Chronic Kidney Disease
CLEM Cardiovascular Life Expectancy Model
DASH Dietary Approaches to Stop Hypertension
DM Diabetes Mellitus
eGFR Estimated Gomerular Filtration Rate
ePARmed-X+ Electronic Physical Activity Readiness Medical Examination
FH Familial hypercholesterolemia
FPG Fasting Plasma Glucose
FRS Framingham Risk Score
HDL–C High Density Lipoprotein Cholesterol
HIV Human Immunodeficiency Virus
hsCRP High-Sensitivity C-Reactive Protein
HTN Hypertension
LDL-C Low Density Lipoprotein Cholesterol
Lp(a) Lipoprotein(a)
NNT Needed to Treat
PAR-Q+ Physical Activity Readiness Questionnaire for Everyone
PCSK9 Proprotein Convertase Subtilisin/Kexin 9
PUFA Poly Unsaturated Fatty Acids
SBIRT Screening, Brief Intervention and Referral to Treatment
TC Total Cholesterol
TG Triglycerides
USPSTF U.S. Preventive Services Task Force
Practitioner Resources
For practitioners, refer to Associated Document: Resource Guide for Practitioners – Tools for Primary Prevention of Cardiovascular Disease.
Canadian Cardiovascular Society Pocket guide: quick-reference tool that features diagnostic and treatment recommendations based on the CCS Dyslipidemia Guidelines (2006, 2009, 2012 and 2016).
RACE: Rapid Access to Consultative Expertise Program – www.raceconnect.ca
A telephone consultation line for select specialty services for physicians, nurse practitioners and medical residents. If the relevant specialty area is available through your local RACE line, please contact them first. Contact your local RACE line for the list of available specialty areas. If your local RACE line does not cover the relevant specialty service or there is no local RACE line in your area, or to access Provincial Services, please contact the Vancouver/Providence RACE line.
- Vancouver Coastal Health Region/Providence Health Care: www.raceconnect.ca
604-696-2131 (Vancouver) or 1-877-696-2131 (toll free) Available Monday to Friday, 8 am to 5 pm - Northern RACE: 1-877-605-7223 (toll free)
- Kootenay Boundary RACE: divisionsbc.ca/kootenay-boundary/race-line 1-844-365-7223 (toll free)
- For Fraser Valley RACE: www.raceapp.ca (download at Apple and Android stores)
- South Island RACE: www.raceapp.ca (download at Apple and Android stores) or see www.divisionsbc.ca/south-island/RACE
Pathways – PathwaysBC.ca
An online resource that allows GPs and nurse practitioners and their office staff to quickly access current and accurate referral information, including wait times and areas of expertise, for specialists and specialty clinics. In addition, Pathways makes available hundreds of patient and physician resources that are categorized and searchable.
General Practice Services Committee – www.gpscbc.ca
- Practice Support Program: offers focused, accredited training sessions for BC physicians to help them improve practice efficiency and support enhanced patient care.
- Chronic Disease Management and Complex Care Incentives: compensates GPs for the time and skill needed to work with patients with complex conditions or specific chronic diseases.
Health Data Coalition: hdcbc.ca
An online, physician-led data sharing platform that can assist you in assessing your own practice in areas such as chronic disease management or medication prescribing. HDC data can graphically represent patients in your practice with chronic kidney disease in a clear and simple fashion, allowing for reflection on practice and tracking improvements over time.
HealthLinkBC: healthlinkbc.ca
HealthLinkBC provides reliable non-emergency health information and advice to patients in BC. Information and advice on managing chronic kidney disease in several languages is available by telephone, website, a mobile app and a collection of print resources. Patients can speak to a health services navigator, registered dietitian, registered nurse, qualified exercise
professional, or a pharmacist.
Patients may call 8-1-1 toll-free in B.C., or for the deaf and the hard of hearing, call 7-1-1.
Diagnostic Codes
Prevention visit code – 14066
Smoking – 786
Unhealthy eating and medical obesity – 783
Physically inactive - 785
Appendices
- Appendix A: Primary Prevention of Cardiovascular Disease Algorithm (PDF, 168KB)
- Appendix B: Framingham 10-year Risk Estimation (PDF, 99KB)
- Appendix C: Lipid Testing in Primary Prevention of Cardiovascular Disease (PDF, 107KB)
- Appendix D: HMG-CoA REductase Inhibitors (Statins) (PDF, 111KB)
Associated Documents
Resource Guide for Physicians - Tools for Primary Prevention of Cardiovascular Disease
This guideline is based on scientific evidence current as of the effective date.
This guideline was developed by the Guidelines and Protocols Advisory Committee in collaboration with the Provincial Laboratory Medicine Services, and adopted under the Medical Services Act and the Laboratory Services Act.
For more information about how BC Guidelines are developed, refer to the GPAC Handbook available at BCGuidelines.ca: GPAC Handbook.
THE GUIDELINES AND PROTOCOLS ADVISORY COMMITTEE
|
The principles of the Guidelines and Protocols Advisory Committee are to:
Contact Information: Guidelines and Protocols Advisory Committee PO Box 9642 STN PROV GOVT Victoria, BC V8W 9P1 Email: hlth.guidelines@gov.bc.ca Website: www.BCGuidelines.ca Disclaimer The Clinical Practice Guidelines (the “Guidelines”) have been developed by the Guidelines and Protocols Advisory Committee on behalf of the Medical Services Commission. The Guidelines are intended to give an understanding of a clinical problem, and outline one or more preferred approaches to the investigation and management of the problem. The Guidelines are not intended as a substitute for the advice or professional judgment of a health care professional, nor are they intended to be the only approach to the management of clinical problem. We cannot respond to patients or patient advocates requesting advice on issues related to medical conditions. If you need medical advice, please contact a health care professional. |


 TOP
TOP