PharmaNet Re-Conformance Project
To All PharmaNet Software Support Organizations:
Action required: Please provide a direct point-of-contact (including the individual’s name, phone, and email) for the Conformance, Integration and Standards (CIS) team to establish communications and scheduling.
Overview
The updated BC Conformance Standards v3.4 replace all previous releases of the compliance/conformance standards.
Note: The PharmaNet Legacy Compliance Standards are no longer available for new development.
All applications must pass conformance testing against the ministry's updated conformance standards in order to integrate with PharmaNet.
There have been significant updates which include (but are not limited to) the following:
- Volume 1: Conformance processes and policy updates.
- Volume 2: Enhanced privacy and security rules that reflect current industry standards (e.g., safeguarding personal health information, allowing access only to authorized individuals).
- Volume 4C: Updated PharmaNet conformance standards.
Highlights include (but are not limited to):
- Transaction matrix updates including the following new transactions (for PharmaNet v70 access types):
- TRX (X0/X5) – Retrieve Patient Prescription
- TRX (X1/X6) – Record Prescription
- TRX (X2/X7) – Update Prescription Status
- TRX (X3/X8) – Adapt Prescription
- TRX (X4/X9) – Retrieve Prescriber Prescription
- Mandatory Global PharmaNet ID (GPID) and IP address in the message header (MSH) security field.
- New applications must integrate with the Client Registry.
Note: Integration with the Client Registry is currently optional for existing applications.
- Updates to:
- Access logging and addition of telepharmacy site reporting;
- Data retention (local business & prescription records);
- Electronic storage of pharmacy and prescription records;
- Mandatory display standards for transactions; and
- Storage of PharmaNet clinical data.
Mandatory Requirements
Each of the following requirements must be completed to integrate with PharmaNet:
- A Vendor Participation Agreement (VPA) must be signed by all organizations.
- All applicable conformance standards (e.g., business rules, application enforced rules, privacy and security rules, training requirements) will be taken in account in the conformance decision.
- All sites must deploy a software version that has passed conformance through the PharmaNet Re-Conformance Project in order to continue in the production environment.
- All previous software versions will be decommissioned following a transition period.
- Additional application changes that follow the PharmaNet Re-Conformance Project will be managed through the normal process as described in Volume 1: Overview & Conformance Processes (PDF, 625KB).
- All previous software versions will be decommissioned following a transition period.
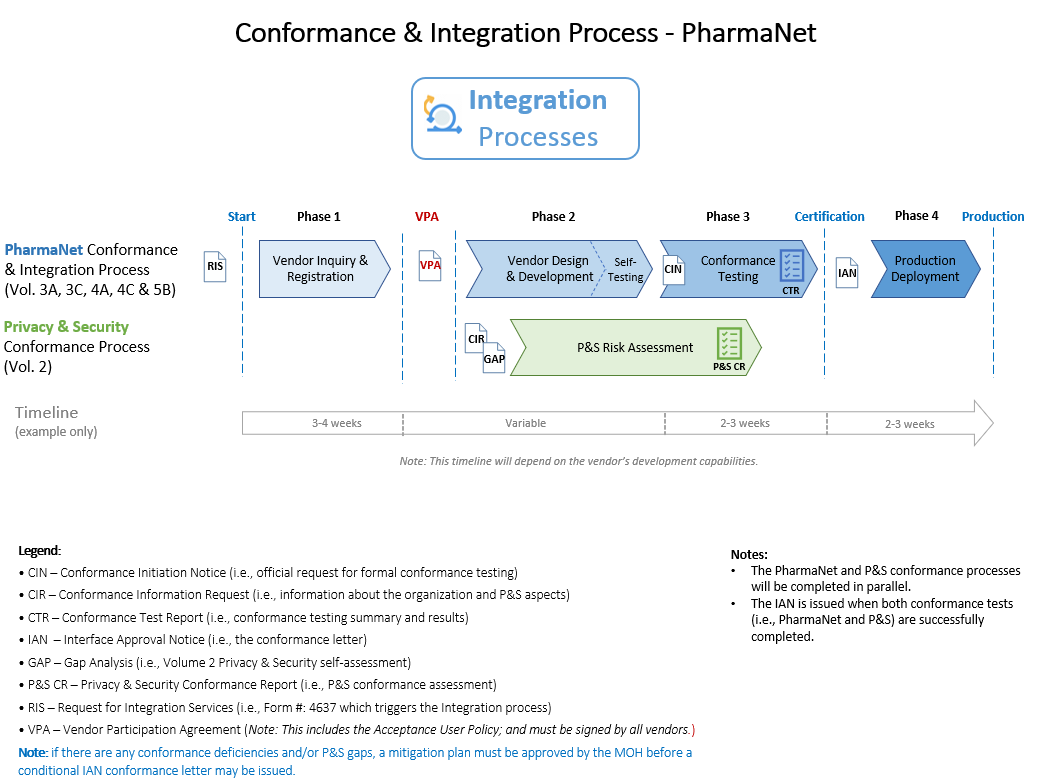
Process
The following is a high-level summary of the ministry’s conformance processes for PharmaNet integration:
- Each organization must sign the VPA.
- Organizations without a VPA cannot integrate with PharmaNet.
- The VPA will replace any existing agreements (e.g., SLA).
- The term of the VPA will be for five years after the date of signing.
- Each organization must pass self-testing.
- Once an organization is ready to proceed with self-testing, the ministry will provide detailed information.
- Once an organization is ready to proceed with self-testing, the ministry will provide detailed information.
- Each organization must submit a Conformance Initiation Notice (CIN) to schedule re-conformance testing.
- The PharmaNet and P&S conformance processes will be completed in parallel.
- All applicable conformance standards (e.g., business rules, application enforced rules, privacy and security rules, training requirements) will be taken in account in the conformance assessment.
- An Interface Approval Notice (IAN) will be issued and allow the application to be released in production for the term specified (including any identified conditions).
Conformance Test Cases
The required connections, test cases, and test data will be provided once an organization is ready to proceed with self-testing and/or formal conformance testing.
Timeline
Conformance testing will be scheduled by access type.
Note: The ministry will attempt to accommodate requests to combine testing for multiple access types.
Contact
For more information, contact the CIS Team at: