Warfarin
Effective Date: January 18, 2023
Recommendations and Topics
- Scope
- Key Recommendations
- Pharmacological Properties
- Therapeutic Indications
- Initiating Warfarin
- Chronic Warfarin Dosing and INR Monitoring
- Bleeding Complications
- Resources
- Appendices
Scope
This guideline provides recommendations for the primary care management of warfarin therapy in adults aged ≥19 years. This guideline describes: 1) initiation, 2) international normalized ratio (INR) monitoring with optimal ranges, and 3) dosage adjustment.
Indications and decision to treat with warfarin, elective interruption, and emergency reversal are all outside the scope of this guideline. Refer to BC Guidelines: Direct Acting Oral Anticoagulants and BC Guidelines: Oral Anticoagulants: Elective Interruption & Emergency Reversal for more information on these topics.
Key Recommendations
- Having a warfarin management plan that includes dosing tools, ongoing patient education and follow-up involving caregivers and other health professionals (e.g., pharmacists, dieticians, nurses) can maximize benefits and safety, and increase adherence.
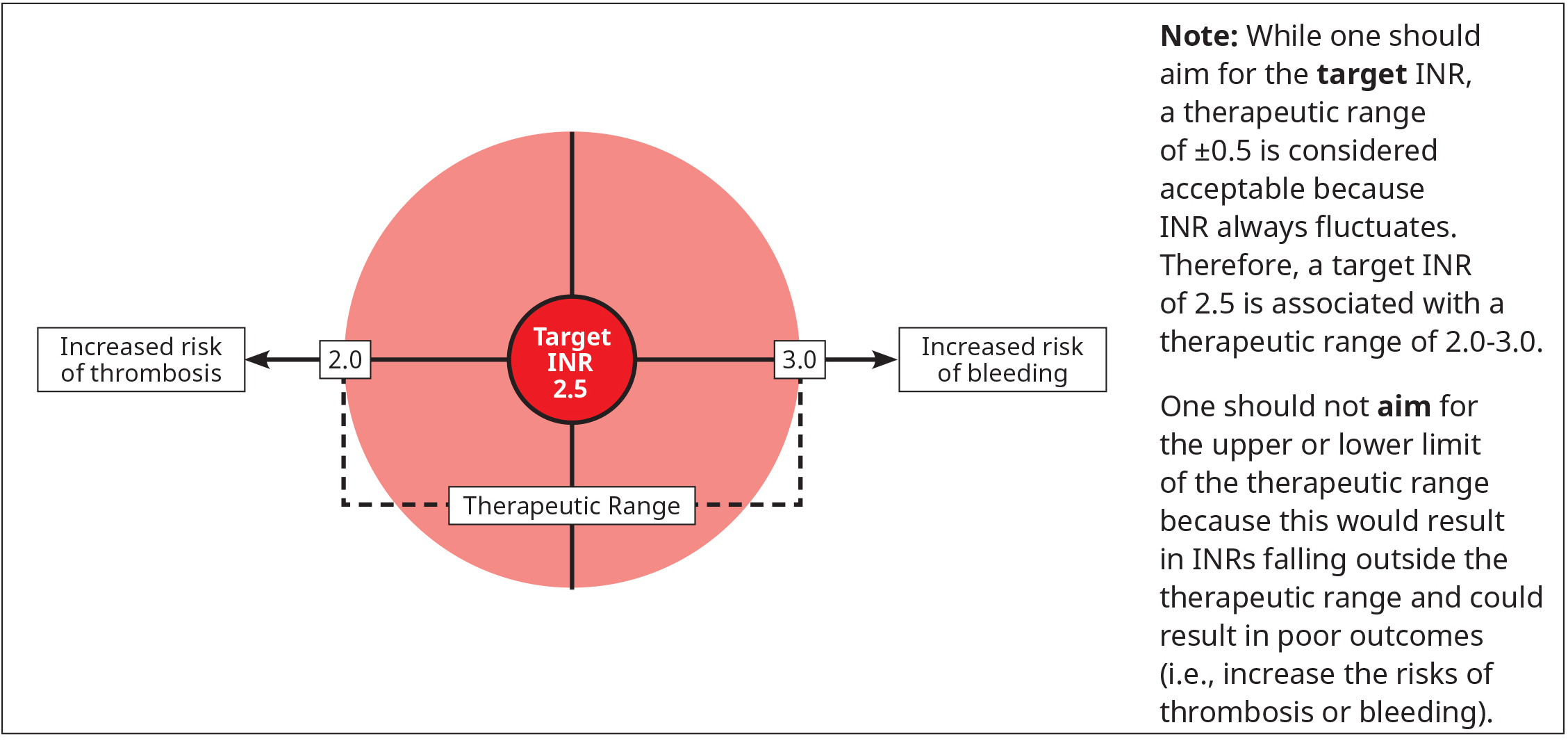
- Warfarin should be dosed to achieve the target INR for a specific indication. This is preferred over accepting values at the outer bounds of the therapeutic range (i.e., target INR ±0.5).
- Consider using a standard dosing nomogram or computerized decision-support software to maximize the probability of achieving the target INR.
- Routine INR monitoring is necessary to measure the achieved anticoagulant effect and determine if dose changes are necessary.
- Because the anticoagulant effect (onset and offset) of warfarin is delayed, dose changes more than twice a week are not recommended during chronic therapy.
- Dose changes are not necessary for transient fluctuations in the INR due to short-term medication use (e.g., antibiotics). INR testing may be warranted within a week of initiating a new medication that has relevant drug-drug interactions with warfarin.
- Warfarin cannot achieve a therapeutic effect within the first five days, regardless of INR. As such, when warfarin is used to treat acute thrombosis, low molecular weight heparin (LMWH) or unfractionated heparin must be used concomitantly for at least five days.
- There are no dietary restrictions for patients taking warfarin. Patients should maintain a regular and consistent diet. This is especially true for vitamin K intake.
Pharmacological Properties
Warfarin is an indirect oral anticoagulant that reduces the activity of vitamin K-dependent coagulation factors II, VII, IX and X, as well as endogenous anticoagulants proteins C and S. Warfarin is almost fully bound to albumin in blood, thus, patients with hypoalbuminemia (e.g., malnourished, liver disorders, post-operative, etc.) require lower doses.
The anticoagulation effect of warfarin is assessed with the INR. Warfarin’s effect on the INR is detectable within 24 hours of administration. However, the full therapeutic effect is not achieved for 5-7 days due to the long half-life of prothrombin (factor II), the key coagulation factor that converts fibrinogen to fibrin.1 Hence, a more rapidly acting anticoagulant, such as LMWH or unfractionated heparin, must be given concomitantly for at least five days when initiating warfarin for acute thrombosis.
Therapeutic Indications
Warfarin effectively reduces the risk of thromboembolic events. It is most commonly used for the prevention of stroke or systemic embolism in patients with atrial fibrillation (AF),2–5 treatment of acute venous thromboembolism (VTE), long-term secondary prevention for VTE, and prevention of thrombotic complications with mechanical heart valves. Warfarin is also sometimes used in arterial thrombotic diseases.
Initiating Warfarin
Dosing tools such as nomograms and computerized or mobile decision support programs have been shown to improve the likelihood of achieving the target INR and the proportion of time that the INR is within the therapeutic range (i.e., target INR ±0.5).
Prior to initiating warfarin treatment:
-
Consider contraindications outlined in Table 1: Examples of absolute and relative contraindications for warfarin. All contraindications are relative to a patient’s risk for thrombosis weighed against the risk for bleeding while on anticoagulation therapy. Refer to Table 4: Risk factors for bleeding complications on anticoagulation therapy for more information on risk factors for bleeding complications on anticoagulation therapy.
-
Establish the baseline INR to guide further therapy and dose adjustments.
-
Counsel the patient and/or caregiver(s) on potential drug-drug and dietary interactions, the importance of INR monitoring, and signs of thrombosis (e.g., leg swelling) and bleeding (e.g., melena). Refer to Appendix A: Important Interactions with Warfarin, Patient Education and Resources sections for more information.
Table 1: Examples of absolute and relative contraindications for warfarin
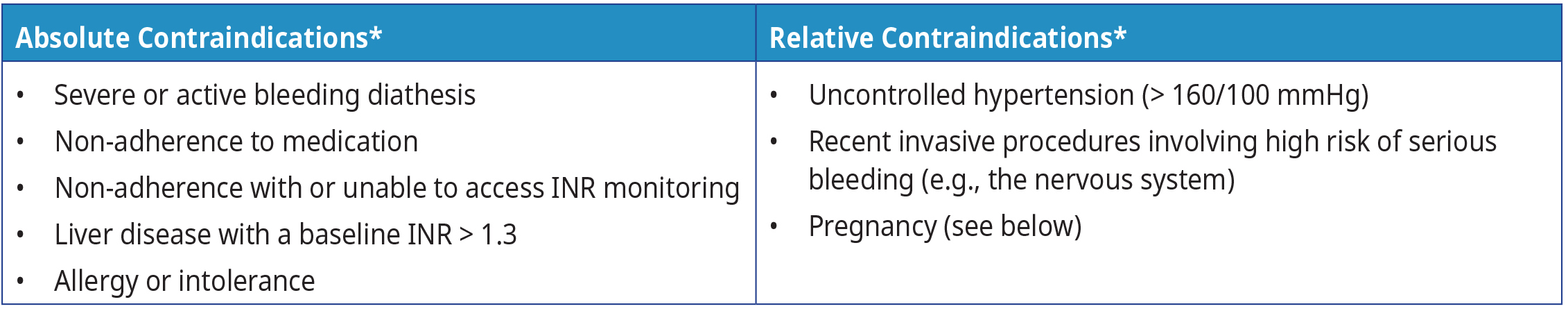 Abbreviations: INR = international normalized ratio; mmHg = millimetres of mercury
Abbreviations: INR = international normalized ratio; mmHg = millimetres of mercury
Footnote: * Refer to the product monograph for a complete list of contraindications.
If possible, warfarin therapy should be avoided during pregnancy because it crosses the placenta.6 Warfarin use during pregnancy is associated with increased risk, including fetal bleeding, teratogenicity and spontaneous abortion.5 LMWH is considered the anticoagulant of choice during pregnancy because it does not cross the placenta.5,6 If warfarin therapy is critical during pregnancy (e.g., for patients with high risk mechanical valves), it should be avoided during the first trimester to reduce the risk of fetal defects and 2-4 weeks before delivery due to the risk of peripartum bleeding.6 Consider consulting a hematologist and an obstetrician for anticoagulated patients who are attempting to conceive or who are already pregnant. Warfarin therapy is not contraindicated during breastfeeding.5
Patient Education
Warfarin is more likely to be used safely by a patient who 1) understands the absolute need for, and agrees to comply with, routine INR monitoring, and 2) is aware of the potential for drug and diet interactions. Encourage patients to discuss specific medication factors with their community pharmacist and refer them to this Thrombosis Canada patient information sheet or HealthLinkBC for additional patient materials.
Incorporate the following key items into your education discussions when initiating warfarin:
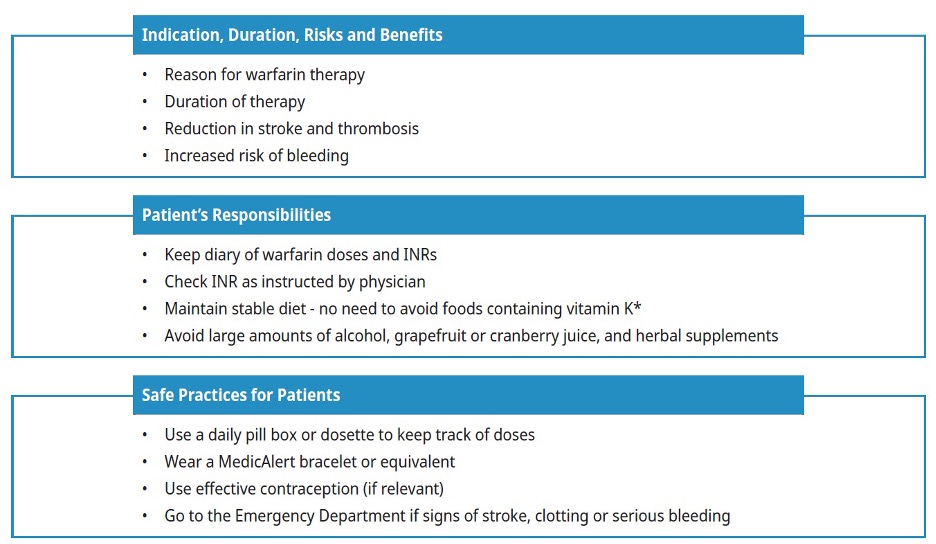
Dietary intake of vitamin K: The effect of warfarin may be inhibited by very high dietary or supplemental intake of vitamin K (e.g., Boost, Ensure). However, it is not necessary to advise patients to avoid or limit vitamin K intake. It is generally recommended that patients on warfarin try to consume the adequate intake for vitamin K (90-120 mcg) while avoiding large fluctuations that might interfere with the adjustment of their anticoagulant dose.5
Initial Dose
The initial dose of warfarin is typically 5 mg/day. However, a lower starting dose (e.g., 1-2 mg daily) may be considered for patients with frailty, >70 years, elevated baseline INR 1.2-1.3, hypoalbuminemia (e.g., malnourished, liver disorders, post- operative), heart failure, recent use of some antibiotics that would impact gut flora, or known to take other medications that increase sensitivity to warfarin.2,5,7 Patients of Asian descent tend to require lower doses, while patients of African American descent tend to need higher doses because of the differences in genetic polymorphisms between these populations.8
Whenever feasible, prescribe a single strength warfarin tablet to increase safety and dose flexibility. Patients should take their warfarin once a day at the same time in the evening and have their INR test performed in the morning. This dosing schedule limits diurnal variations and provides the primary care provider with a same day window for dosage adjustment in the event of an unanticipated INR change.
Chronic Warfarin Dosing and INR Monitoring
Patient and caregiver education is essential to maximize adherence and maintain therapeutic stability. A warfarin management plan that includes dosing tools, ongoing patient education and follow-up involving caregivers and other health professionals (e.g., pharmacists, dieticians, nurses), can maximize benefits and safety, increase adherence, and improve therapeutic stability.
Target INR and Acceptable Therapeutic Ranges
Warfarin is dosed to achieve a target INR of 2.5 for most patients,1,9 though this target differs for patients with prosthetic valves (see Table 2: Target INR for patients with prosthetic valves) and refractory hypercoagulable states (e.g., recurrent thrombosis while on anticoagulant therapy). Common indications with a target INR of 2.5 are VTE (acute and long-term therapy), nonvalvular AF, and triple-positive antiphospholipid syndrome.
Subtherapeutic INRs are associated with increased risk of thrombosis while supratherapeutic INRs are associated with increased risk of bleeding. Avoid subtherapeutic anticoagulation because not only does it provide suboptimal protection against thrombosis, but it also increases the risk of bleeding from baseline.
Figure 1: Target INR for most therapeutic indications
Target INR and duration of anticoagulation with warfarin in patients with prosthetic valves vary depending on several factors, including type of valve, risk of bleeding and if there are other conditions that increase the risk of stroke or systemic thromboembolism. See Table 2: Target INR for patients with prosthetic valves for more information. Consider seeking specialist advice for most updated recommendations.
Table 2: Target INR for patients with prosthetic valves
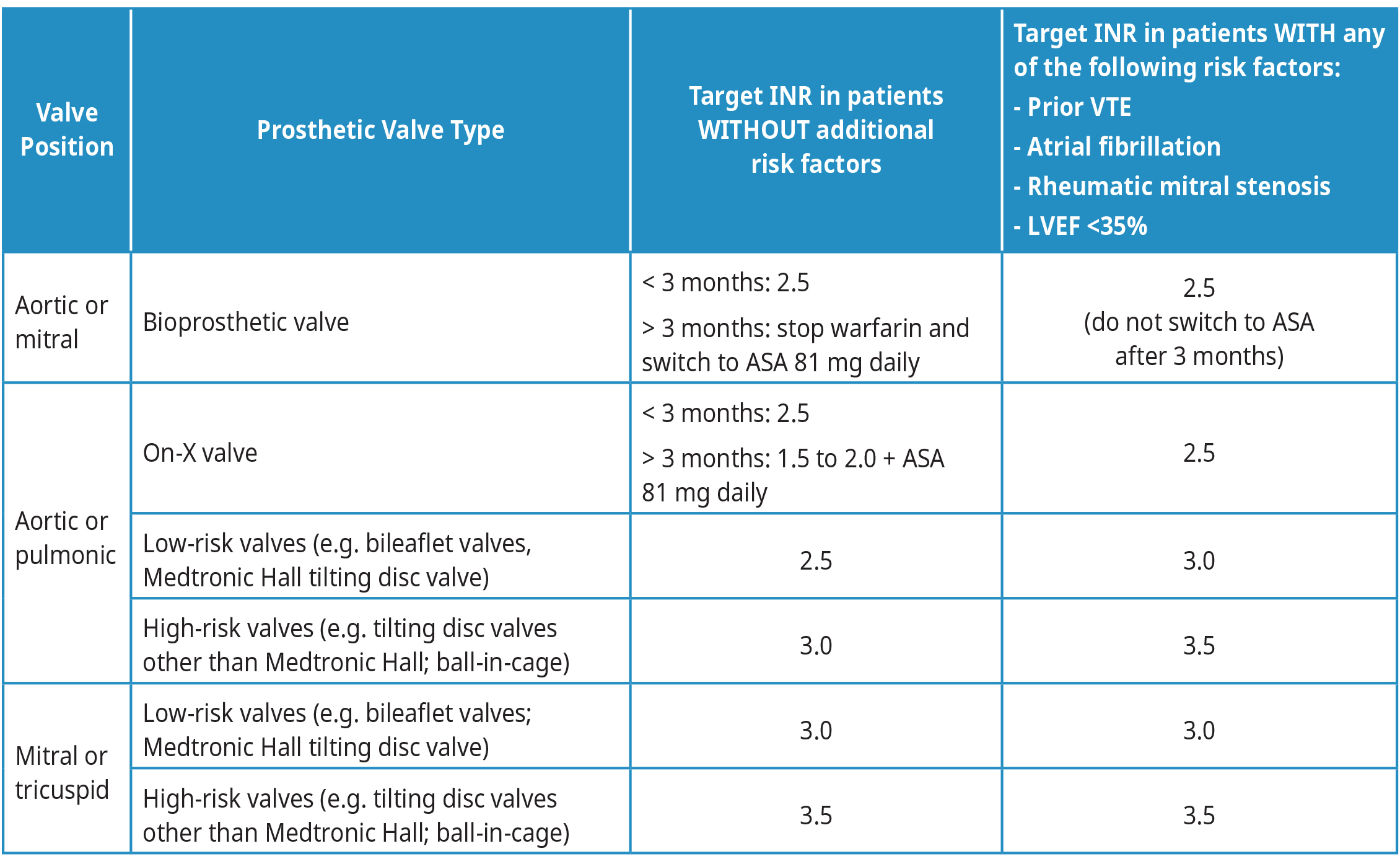
Abbreviations: LVEF = left ventricular ejection fraction; VKA = Vitamin K Antagonist (e.g, warfarin).
Adapted from: 2020 American College of Cardiology and American Heart Association Guidelines for the Management of Patients with Valvular Heart Disease.10
INR Monitoring
Warfarin's narrow therapeutic index necessitates close and long-term monitoring. Patients must undergo routine INR monitoring to measure the achieved anticoagulant effect, with increased testing frequency required at the time of drug initiation and periods of dose adjustments. The INR rises without concomitant therapeutic anticoagulant effect during the first 5-7 days of treatment. During the chronic or maintenance phase, a dose change may not be reflected in INR for 4-5 days and so dose changes more than twice a week are not recommended. See Figure 2: Recommended INR monitoring frequency for a suggested monitoring schedule and the Dose Adjustments section below for more information.
Figure 2: Recommended INR monitoring frequency
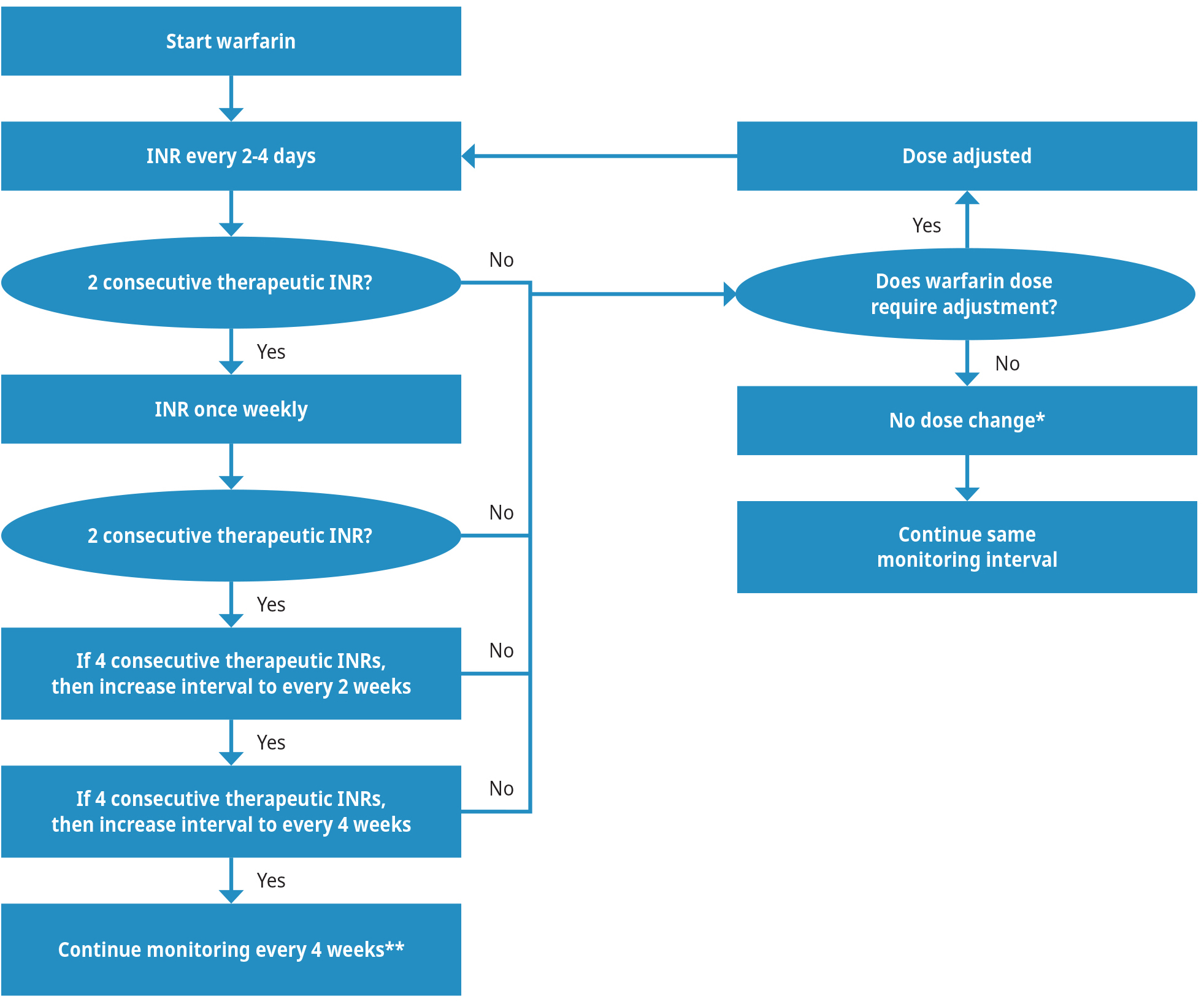
Note: INR test frequency may need to increase if any of the following occurs during chronic/maintenance therapy:
- Consecutive non-therapeutic INRs
- Intercurrent gastrointestinal illness
- Change in some medications or herbal supplements (Appendix A: Important Interactions with Warfarin)
- Significant changes in diet
- Dose adjustments are not necessarily required if the INR is sub/supratherapeutic due to:
- Patient non-adherence (e.g., patient forgot doses or took too many doses)
- < 5 days since last dose change
- Binge alcohol use (> 1-2 drinks will transiently elevate INR)
** In a small group of very stable patients (i.e., stable INRs and no dosage change for 3 months), INR can be monitored as infrequently as once every 12 weeks.
Dose Adjustments
The optimal chronic or maintenance dose for warfarin varies from patient to patient and at different times in the same patient.1,5,9 There is no maximal or minimal dose to maintain a therapeutic effect. The actual dose is not important and can range from 0.5-20 mg or more daily.
Dose adjustment is not required for minor fluctuations of INR if the patient remains within their therapeutic INR range. Fluctuations of INR beyond therapeutic range should always prompt direct communication with the patient to determine cause.
One common approach to fluctuating INR is to change the total weekly warfarin dose.11 For example, if the patient is taking 5 mg/day, the weekly dose is 35 mg. If the dose must be decreased by 10%, then the weekly dose should be 35 mg – 3.5 mg = 31.5 mg and the daily dose is 31.5 mg/7 = 4.5 mg. Refer to Table 3: Warfarin dose adjustments for more detailed information.
Table 3: Warfarin dose adjustments. Refer to Figure 2 for INR test timing.
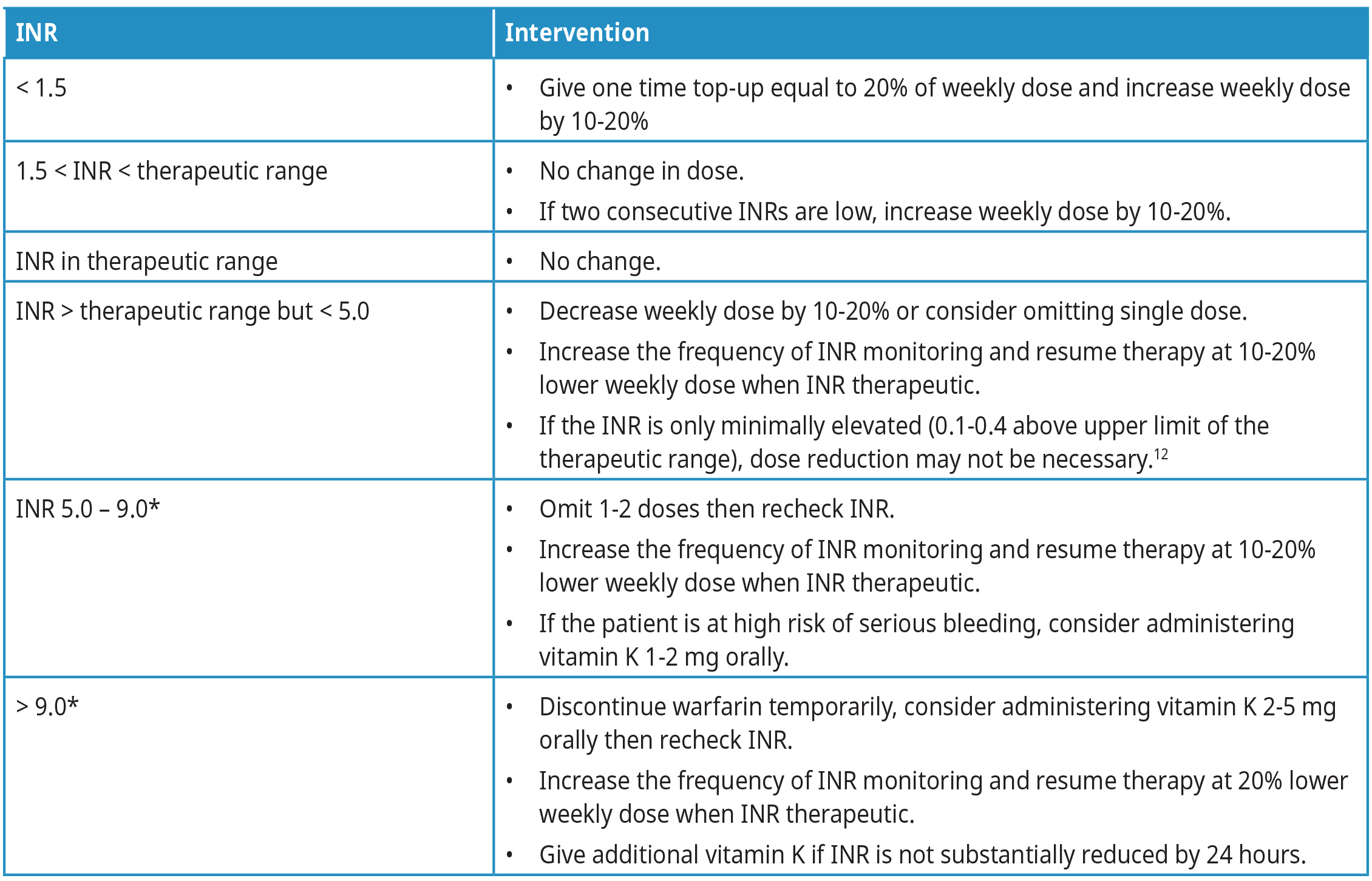 Abbreviations: INR = international normalized ratio.
Abbreviations: INR = international normalized ratio.
*Bleeding risk increases exponentially from INR 5 to 9 and should be monitored closely.
Use of Vitamin K
Oral vitamin K (1-2 mg) can be used to lower a high INR (INR > 5.0) in patients with a high risk of bleeding.5,9,13 Refer to Table 3: Warfarin dose adjustments for dosing information. Avoid subcutaneous vitamin K because absorption is unpredictable. Also, do not give intramuscular vitamin K as it increases the risk of intramuscular hemorrhage. Intravenous vitamin K achieves the fastest correction but can cause facial flushing, diaphoresis, chest pain, hypotension, dyspnea, and, rarely, anaphylaxis, so should be given only in emergency situations and by slow infusion,13 or when oral absorption is likely impaired (e.g., biliary obstruction, malabsorptive syndromes).
The effect of vitamin K on INR is usually observed within 24 hours after oral administration and within 6-8 hours after intravenous administration.13,14 Patients who have received vitamin K, particularly parenteral doses above 5-10 mg, may be difficult to re-anticoagulate. Therefore, vitamin K doses should be kept as low as possible.
Most outpatient pharmacies do not carry vitamin K because an oral formulation is no longer available in Canada. Sending the patient to an urgent care or emergency department is often the fastest route, where an oral dose of the parenteral preparation in juice or water can be administered.
Bleeding Complications
Bleeding is the most serious complication of warfarin therapy.15–17 The risk of bleeding is influenced by the concomitant use of certain medications (e.g., antiplatelet drugs), patient co-morbidities and lifestyle. Refer to Table 4: Risk factors for bleeding complications on anticoagulation therapy for more information on risk factors for bleeding complications on anticoagulation therapy.
Table 4: Risk factors for bleeding complications on anticoagulation therapy1,17,18
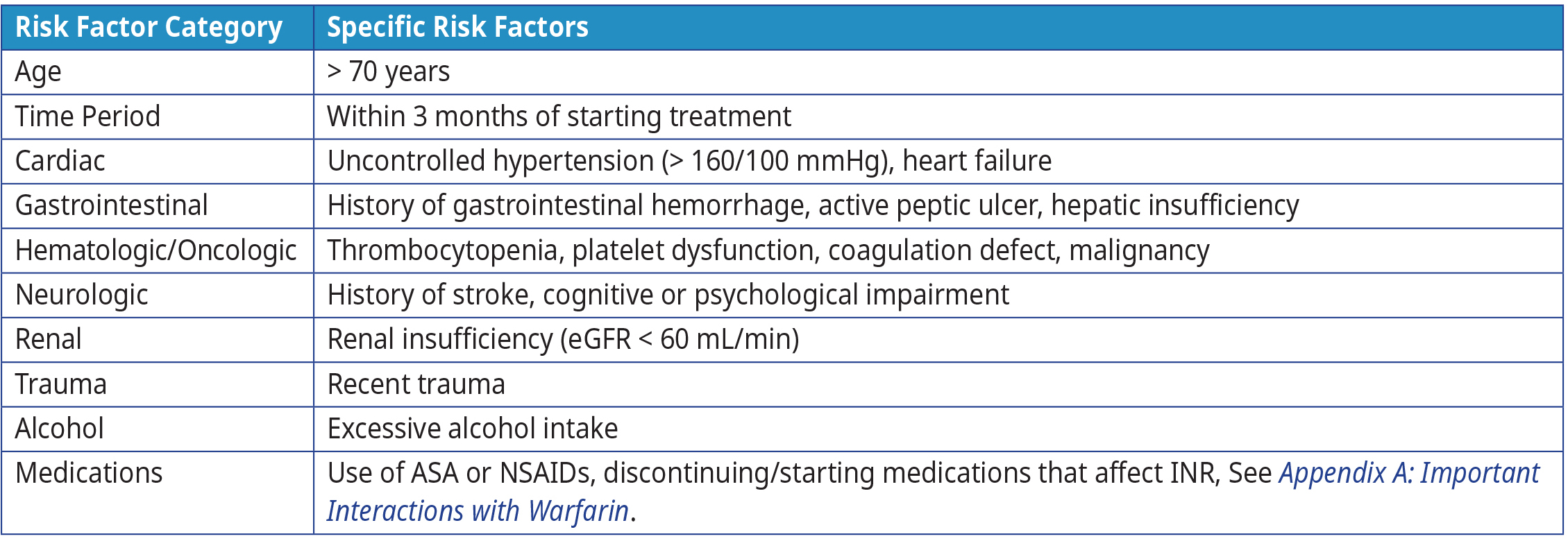 Abbreviations: ASA = acetylsalicylic acid; INR = international normalized ratio; mmHg = millimetres of mercury, mL = millilitre; NSAIDS = nonsteroidal anti-inflammatory drugs.
Abbreviations: ASA = acetylsalicylic acid; INR = international normalized ratio; mmHg = millimetres of mercury, mL = millilitre; NSAIDS = nonsteroidal anti-inflammatory drugs.
Warfarin Reversal Position Statement, Australasian Society of Thrombosis & Haematosis19
While major bleeding is uncommon (1-2% per year), minor bleeding occurs in 10-20% patients on warfarin annually.5 An underlying cause of bleeding should always be sought, especially if the INR is within the therapeutic range or lower. The most common sites of major bleeding are the gastrointestinal tract and genitourinary tract, while most major bleeding is associated with trauma-related or post-operative wounds.15
Heavy menstrual bleeding is sometimes reported in females with reproductive potential and can lead to severe iron deficiency. Iron replacement is recommended if the ferritin confirms iron deficiency (see BC Guidelines: Iron Deficiency). Referral to a gynecologist is recommended to determine if local measures or systemic therapies are necessary or useful to reduce heavy flow. For this and other non-critical bleeding (e.g., recurrent epistaxis), patient should be encouraged to keep a record of bleeding frequency and severity to help guide management.
Bleeding with a high potential for complications (e.g., elderly, propensity to fall, previous history) requires clinical judgement
to determine whether to manage within the office setting or to send to an acute care facility. Prothrombin complex concentrate and vitamin K are required for emergency reversal of warfarin in situations of potentially life threatening hemorrhage, or when an urgent or emergent procedure must be performed. Refer to BC Guidelines: Oral Anticoagulants: Elective Interruption & Emergency Reversal for more information.
References
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and Management of the Vitamin K Antagonists. Chest. 2008;133(6):160S-198S. doi:10.1378/chest.08-0670.
- Garcia D, Regan S, Crowther M, Hughes RA, Hylek EM. Warfarin Maintenance Dosing Patterns in Clinical Practice. Coron Artery Dis.:8.
- Srivastava A, Hudson M, Hamoud I, Cavalcante J, Pai C, Kaatz S. Examining warfarin underutilization rates in patients with atrial fibrillation: Detailed chart review essential to capture contraindications to warfarin therapy. Thromb J. 2008;6(1):6. doi:10.1186/1477-9560-6-6.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic Therapy to Prevent Stroke in Patients Who Have Nonvalvular Atrial Fibrillation. Ann Intern Med. 2007;146(12):857-867.
- Warfarin. Thrombosis Canada; 2021. https://thrombosiscanada.ca/clinicalguides/#
- Abadi S, Einarson A, Koren G. Use of warfarin during pregnancy. Can Fam Physician. 2002;48:695-697.
- Ageno W, Steidl L, Ultori C, et al. The initial phase of oral anticoagulation with warfarin in outpatients with deep venous thrombosis: Blood Coagul Fibrinolysis. 2003;14(1):11-14. doi:10.1097/00001721-200301000-00003.
- Kaye JB, Schultz LE, Steiner HE, Kittles RA, Cavallari LH, Karnes JH. Warfarin Pharmacogenomics in Diverse Populations. Pharmacother J Hum Pharmacol Drug Ther. 2017;37(9):1150-1163. doi:10.1002/phar.1982.
- Keeling D, Baglin T, Tait C, et al. Guidelines on oral anticoagulation with warfarin - fourth edition: Guideline. Br J Haematol. 2011;154(3):311-324. doi:10.1111/j.1365-2141.2011.08753.x.
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72-e227. doi:10.1161/CIR.0000000000000923.
- Crowther MA, Warkentin TE. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood. 2008;111(10):4871-4879. doi:10.1182/blood-2007-10-120543.
- Banet GA, Waterman AD, Milligan PE, Gatchel SK, Gage BF. Warfarin Dose Reduction vs Watchful Waiting for Mild Elevations in the International Normalized Ratio*. Chest. 2003;123(2):499-503. doi:10.1378/chest.123.2.499.
- Watson HG, Baglin T, Laidlaw SL, Makris M, Preston FE. A comparison of the efficacy and rate of response to oral and intravenous Vitamin K in reversal of over-anticoagulation with warfarin: Vitamin K in Warfarin Reversal. Br J Haematol. 2001;115(1):145-149. doi:10.1046/j.1365-2141.2001.03070.x.
- Pendry K, Bhavnani M, Shwe K. The use of oral vitamin K for reversal of over-warfarinization: Correspondence. Br J Haematol. 2001;113(3):839-842. doi:10.1046/j.1365-2141.2001.02804.x.
- Beyth RJ. Hemorrhagic complications of oral anticoagulant therapy. Clin Geriatr Med. 2001;17(1):49-56. doi:10.1016/S0749-0690(05)70105-1.
- Dahri K, Loewen P. The risk of bleeding with warfarin: A systematic review and performance analysis of clinical prediction rules. Thromb Haemost. 2007;98(11):980-987. doi:10.1160/TH07-04-0297.
- Schulman S, Beyth RJ, Kearon C, Levine M N. Hemorrhagic complications of anticoagulant and thrombolytic treatment. Am Coll Chest Physicians Evid-Based Clin Pract Guidel 8th Ed. 2008;133(6):257S-298S.
- Maddali S, Biring T, Kopecky S, et al. Health Care Guideline: Antithrombotic Therapy Supplement. Institute for Clinical Systems Improvement; 2013. Accessed May 3, 2022. https://vdocuments.site/health-care-guideline-antithrombotic-therapy-supplement-2013-05-30-health-care.html
- Baker RI, Coughlin PB, Gallus AS, Harper PL, Salem HH, Wood EM. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. 2004;181(9):6.
Resources
- Thrombosis Canada for patient information and dosing tools (e.g., nomograms and computerized decision-support software programs).
- HealthLink BC for more information on warfarin and vitamin K.
- BC Guidelines: Oral Anticoagulants: Elective Interruption & Emergency Reversal
- BC Guidelines: Direct Acting Oral Anticoagulants
- BC Guidelines: Stroke and Transient Ischemic Attack
- BC Guidelines: Atrial Fibrillation
- RACE: Rapid Access to Consultative Expertise Program: RACE means timely telephone advice from specialist for Physicians, Medical Residents, Nurse Practitioners, Midwives, all in one phone call.
- Monday to Friday 0800 – 1700
- Online at www.raceapp.ca or though Apple or Android mobile device.
- Local Calls: 604–696–2131 | Toll Free: 1–877–696–2131
- For a complete list of current specialty services visit the Specialty Areas page.
- PathwaysBC: An online resource that allows general and nurse practitioners and their office staff to quickly access current and accurate referral information, including wait times and areas of expertise, for specialists and specialty clinics.
- Health Data Coalition: An online, physician-led data sharing platform that can assist you in assessing your own practice in areas such as chronic disease management or medication prescribing. HDC data can graphically represent patients in your practice with chronic diseases in a clear and simple fashion, allowing for reflection on practice and tracking improvements over time.
- General Practice Services Committee:
- Practice Support Program: offers focused, accredited training sessions for BC physicians to help improve practice efficiency and support enhanced patient care.
- Chronic Disease Management and Complex Care Incentives: compensates GPs for the time and skill needed to work with patients with complex conditions or specific chronic diseases.
Abbreviations
ASA Acetylsalicylic Acid
eGFR Estimated Glomerular Filtration Rate
INR International Normalized Ratio
LMWH Low Molecular Weight Heparin
NSAIDs Nonsteroidal Anti-Inflammatory Drugs
VTE Venous thromboembolism
Appendices
Associated Documents
This guideline is based on scientific evidence current as of the Effective Date.
This guideline was developed by the Guidelines and Protocols Advisory Committee, approved by the British Columbia Medical Association, and adopted by the Medical Services Commission.
For more information about how BC Guidelines are developed, refer to the GPAC Handbook available at BCGuidelines.ca: GPAC Handbook.
The principles of the Guidelines and Protocols Advisory Committee are to:
Contact InformationGuidelines and Protocols Advisory Committee E-mail: hlth.guidelines@gov.bc.ca Web site: www.BCGuidelines.ca Disclaimer The Clinical Practice Guidelines (the “Guidelines”) have been developed by the Guidelines and Protocols Advisory Committee on behalf of the Medical Services Commission. The Guidelines are intended to give an understanding of a clinical problem, and outline one or more preferred approaches to the investigation and management of the problem. The Guidelines are not intended as a substitute for the advice or professional judgment of a health care professional, nor are they intended to be the only approach to the management of clinical problem. We cannot respond to patients or patient advocates requesting advice on issues related to medical conditions. If you need medical advice, please contact a health care professional. |


 TOP
TOP