Heart Failure - Diagnosis and Management
Effective Date: July 26, 2023
Recommendations and Topics
- Scope
- Key Recommendations
- Definition
- Epidemiology
- Diagnosis
- Management
- Reassessment and Follow-up
- Indications for Referral
- Palliative Care and End of Life Care
- Resources
This guideline provides recommendations for the diagnosis and management of people aged ≥ 19 years with suspected or confirmed heart failure in the ambulatory primary care setting. Given that heart failure is associated with reduced life expectancy, advanced care planning and palliative care are also addressed.
For specific guidance on heart failure in acute care settings refer to the Canadian Cardiovascular Society Heart Failure guidelines.
- Echocardiogram (ECHO) is the gold standard for assessing structural and/or functional cardiac abnormalities and determining ejection fraction to guide treatment planning.
- BNP and NT-proBNP tests have a high sensitivity for the detection of heart failure (HF) and can help with diagnosis while waiting for echocardiogram.
- There are cornerstone therapies that improve clinical outcomes (e.g., reduced hospitalizations, and mortality, improved quality of life) in patients with HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF) necessitating their rapid implementation in eligible patients.
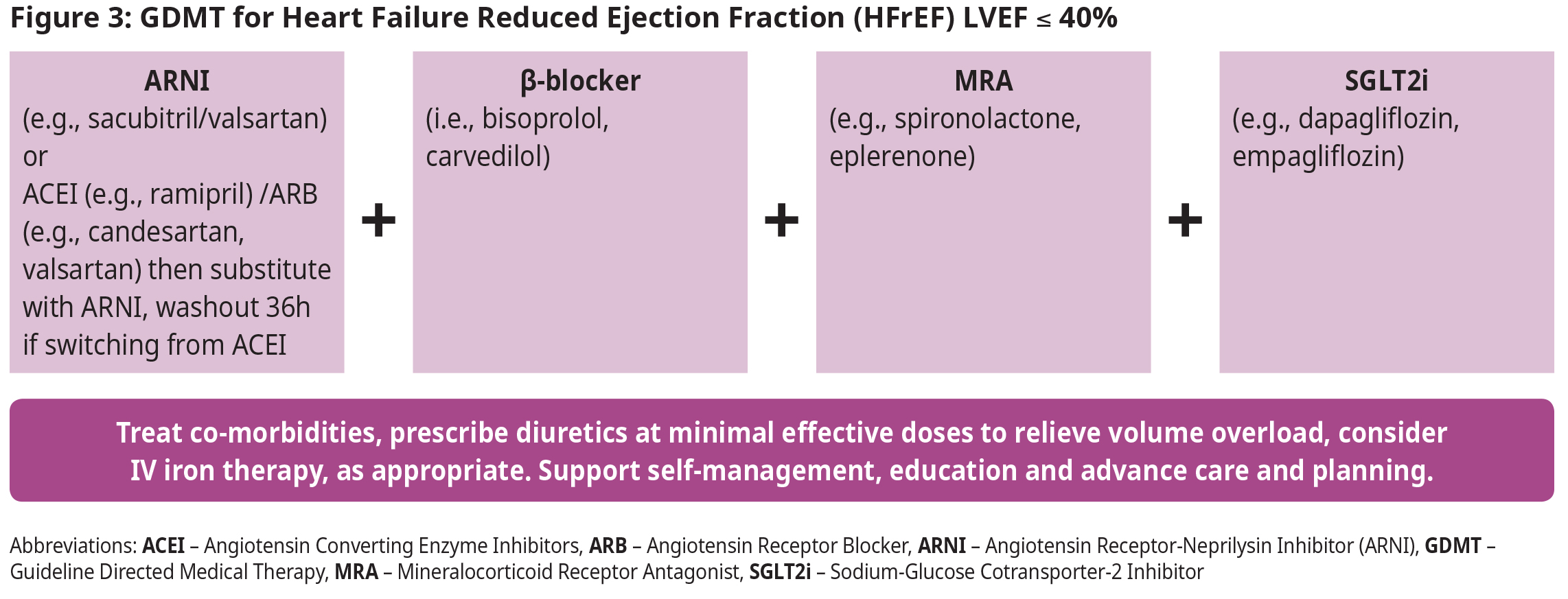
- NEW: It is recommended that in the absence of contraindications, patients with HFrEF be treated with combination therapy including one medication from each of the following categories: Angiotensin receptor-neprilysin inhibitors (ARNI) [or Angiotensin-converting enzyme inhibitors (ACEI)/Angiotensin receptor blockers (ARB)]; beta-blockers (β-blockers); Mineralocorticoid receptor antagonists (MRA); and Sodium-glucose cotransporter-2 inhibitors (SGLT2i). These four medications should be initiated and titrated in parallel as quickly as possible.
- Patient and caregiver education about the importance of self-monitoring and early identification of disease decompensation is crucial to avoid hospitalization and to inform acute treatment.
- Consider referral to a heart function clinic or a multi-disciplinary chronic disease management clinic, where available.
- Initiate advance care planning discussions early in the disease course.
- Incorporate a palliative approach as the disease progresses and/or as per patient preference.
The new universal definition of heart failure (HF):
- Signs and/or symptoms caused by a structural and/or functional cardiac abnormality based on ECHO findings AND
- Elevated natriuretic peptide levels (BNP or NT-proBNP*) OR objective evidence of cardiogenic, pulmonary or systemic congestion.1
* B -Type natriuretic peptide or N‐terminal pro B‐type natriuretic peptide prohormone of BNP
Epidemiology
According to 2020/2021 data,† 2% of the population in BC is affected by HF (prevalence of 137,082), with 18,512 new cases (incidence) in 2020/21.
† British Columbia Ministry of Health [data provider]. BC Observatory for Population and Public Health [publisher]. Chronic Disease Dashboard. Available at: http://www.bccdc.ca/health-info/disease-system-statistics/chronic-disease-dashboard
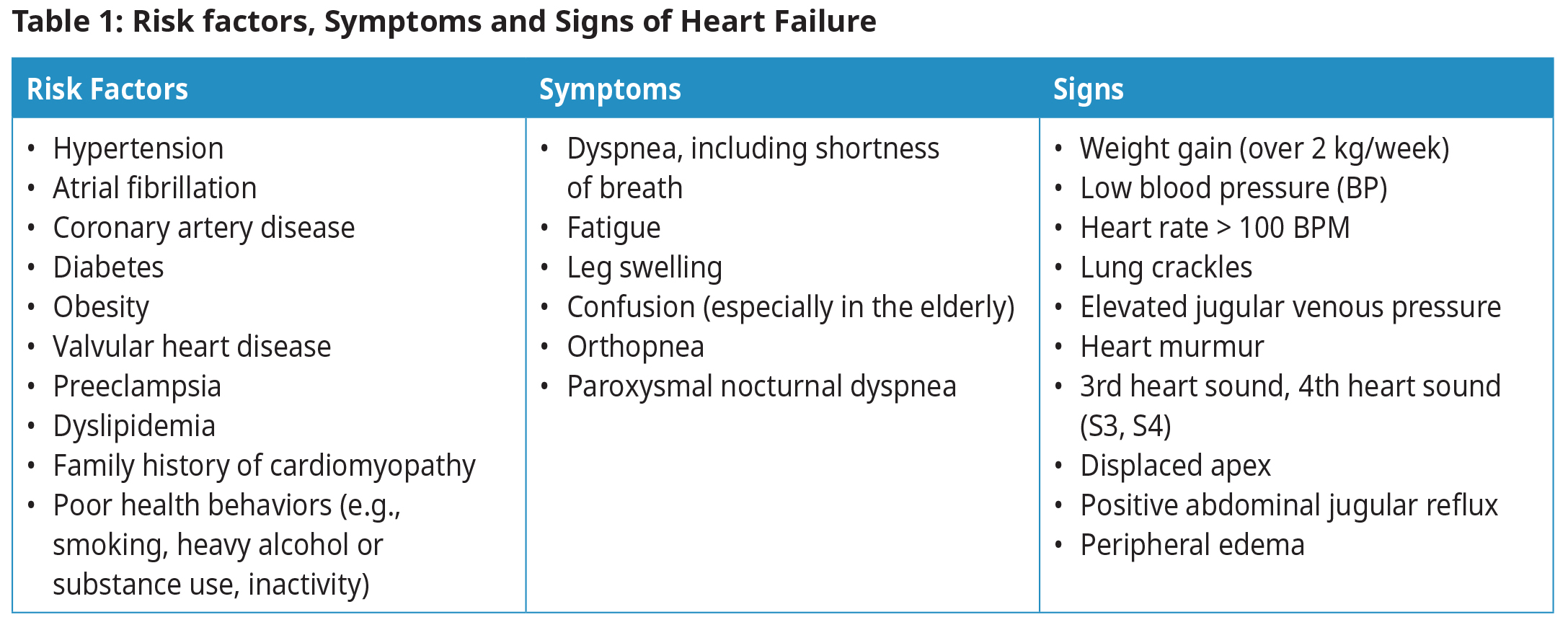
Diagnosis
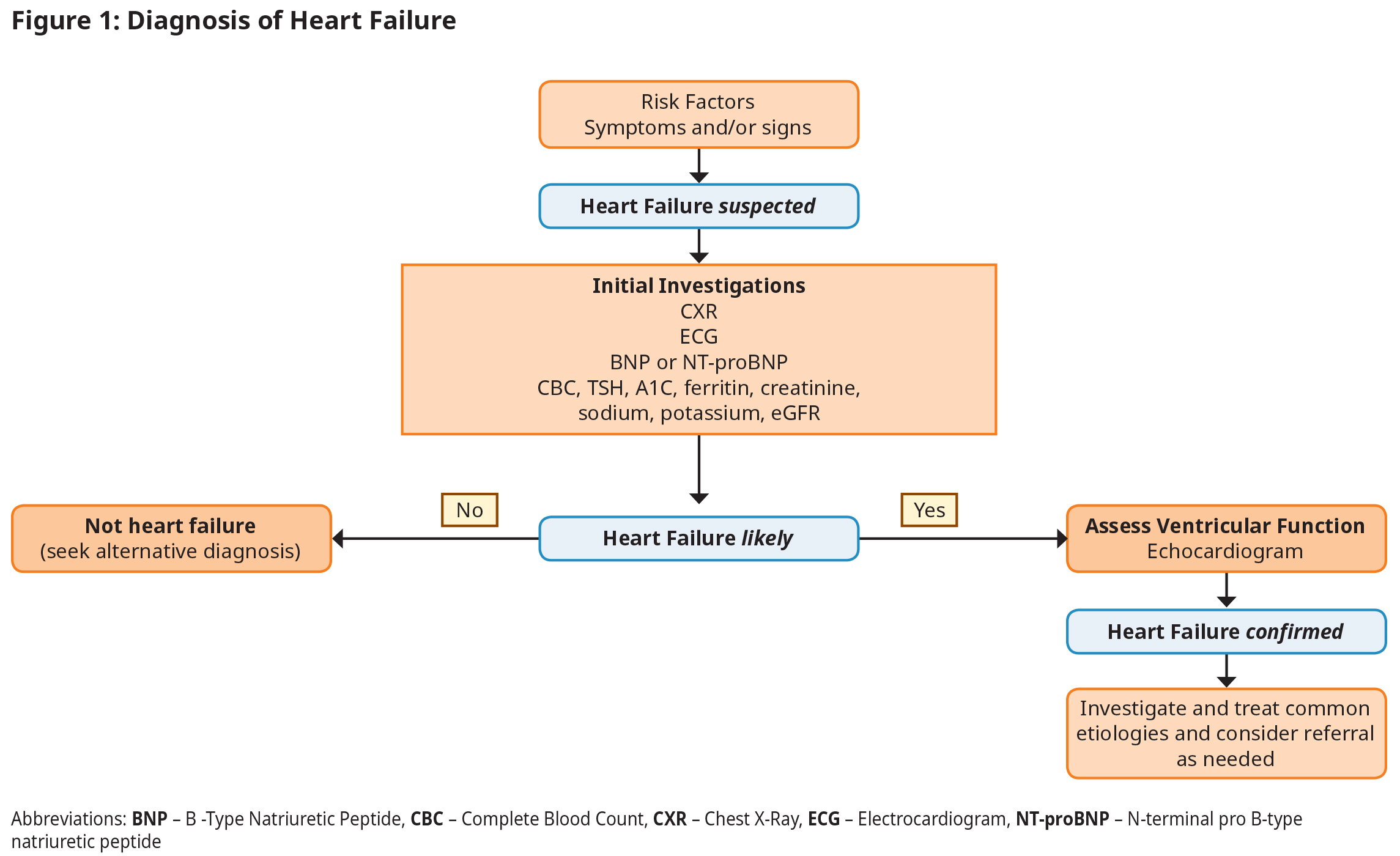
Diagnosis begins with a suspicion of HF based on clinical history (risk factors, symptoms) and physical examination, and is then confirmed with natriuretic peptide testing and ECHO.2,3 See Figure 1: Diagnosis of Heart Failure.
Edema, fatigue, and dyspnea are typical but not specific symptoms of HF; atypical presentations may need to be considered, particularly for women, elderly and obese patients.4,5
Initial investigations should be targeted to confirm or exclude HF, and to identify systemic disorders that might affect its development or progression (e.g., thyroid dysfunction).
Common etiologies for heart failure include tachyarrhythmia, valvular disease, coronary artery disease, and left ventricular hypertrophy. There are several less common etiologies.
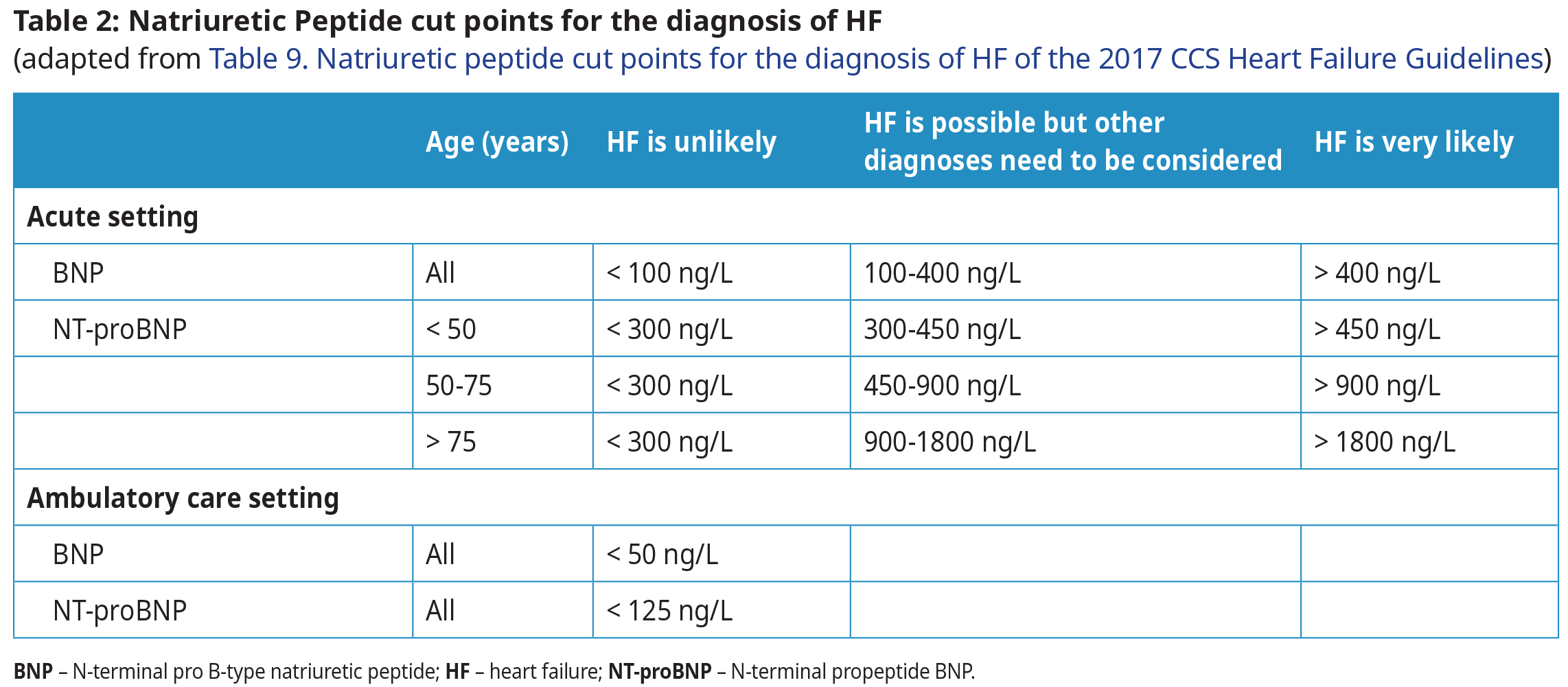
N‐terminal pro B‐type Natriuretic Peptide Testing
- Both BNP and NT-proBNP tests have a high sensitivity and clinical utility for the detection of HF and similar clinical utility. In BC, the laboratory determines which test is performed. In most instances in this guideline, the terms BNP and NT-proBNP are used interchangeably.
- An abnormally high BNP test result does not eliminate the need for cardiac imaging, but in many cases it may allow treatment to begin while waiting for ECHO.
- Interpretation of BNP values differ significantly depending on acute versus ambulatory settings.
- In the ambulatory setting, even a mildly elevated BNP (BNP > 50, NTproBNP > 125 ng/L) indicates need for further investigation and treatment.
- BNP is NOT recommended to screen asymptomatic patients without risk factors, or for interval monitoring of disease severity or therapeutic response.
Echocardiography
ECHO should be performed in all patients with suspected HF to assess cardiac structure, quantify systolic and diastolic function, and to facilitate treatment planning and prognostication. There is regional variation in timely access to ECHO in BC. Delays in obtaining ECHO should not delay initiation of treatment in cases where HF is likely.
Though not a replacement for ECHO, an estimate of left ventricular function may be obtained through alternate imaging modalities:
- Thallium and Sestamibi (MIBI) scan – especially in those patients where ischemia may be the underlying etiology.
- Multi Gated Acquisition Scan (MUGA) or radionuclide angiography – especially for patients with chronic obstructive pulmonary disease or obesity, which may affect ECHO image quality.
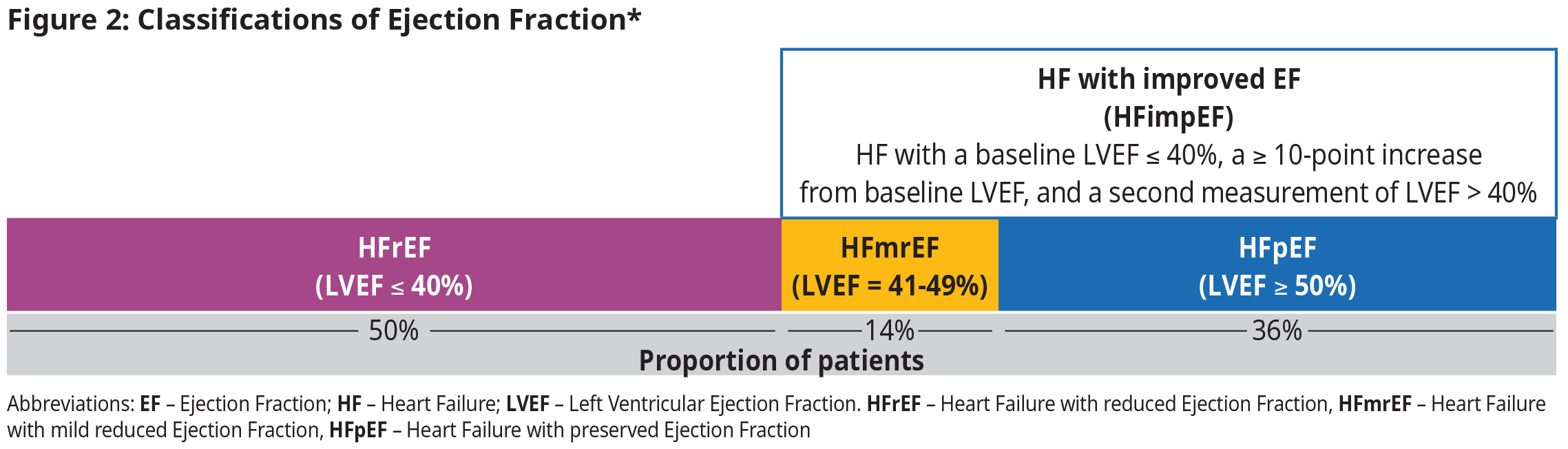
Ejection Fraction
Left ventricular ejection fraction (LVEF) is the primary measure of left ventricular systolic function. Normal LVEF is approximately 60%. Management of HF is based upon LVEF.
 *Adapted from Bozkurt B et al. Eur J Heart Fail. 2021;23:352
*Adapted from Bozkurt B et al. Eur J Heart Fail. 2021;23:352
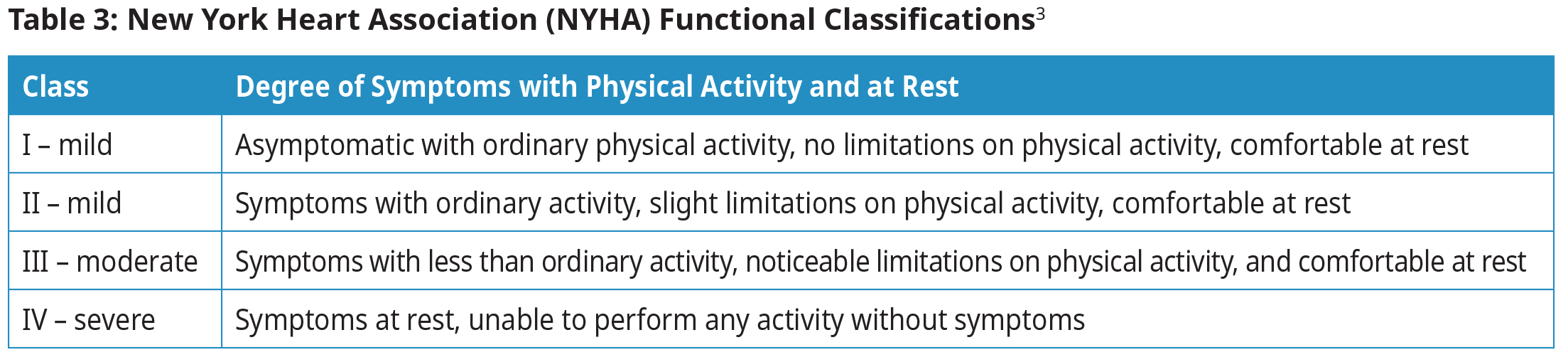
New York Heart Association (NYHA) Functional Classifications
Determine NYHA functional classification in order to guide therapy and determine prognosis.
Management
Patients with HF benefit from frequent assessments and continuity of care, ideally with multi-disciplinary support.
Self-Monitoring and Health Behavior Changes6
HF care may benefit from the patient and their caregiver’s understanding of, and participation in, optimal care in a culturally safe context. Educate the patient and their caregiver(s) on health behavior changes (see Appendix A: Health Behavior Modifications and Self-monitoring Recommendations), HF Zones (see Appendix B: Heart Failure Zones Reference Guide), and medications (see HealthLinkBC: Heart Failure Medications, RxFiles: SADMANS medications in heart failure). See Patient, Family and Caregiver Resources section for a list of patient resources.
Pharmacologic Management
For all patients with HF regardless of LVEF:
- Initiate appropriate treatment for HF (even while awaiting ECHO).
- Once an ECHO is completed, treatment is guided by LVEF.
- Consider diuretic therapy to relieve volume overload and for symptom management.
- Consider initiation of a sodium-glucose cotransporter-2 inhibitor (SGLT2i) and mineralocorticoid receptor antagonist (MRA) to improve cardiovascular outcomes.
- If recently discharged from hospital or experiencing worsening HF symptoms, rapid initiation and up-titration of guideline directed medical therapy (GDMT) reduces cardiovascular mortality and future HF hospitalization.
- GDMT refers to the combination pharmacotherapy used for the treatment of patients with HFrEF and HFpEF.
- Those who fall under the HFimpEF category have a 56% decrease in mortality risk and reduction in hospitalizations.5,7 However, there is evidence of relapse when GDMT is reduced or eliminated, so continuing with GDMT is recommended.8
- Treat co-morbidities like atrial fibrillation, hypertension, diabetes mellitus, renal disease, and sleep apnea based on established BCGuidelines.ca.
- Review medications for intended and unintended effects (e.g., polypharmacy, drug-drug interactions, and aggravation of co-morbid conditions).
- Although studies demonstrating the benefits of HF therapy did not include frail elderly patients, age alone should not preclude attempts to optimize GDMT.9
Heart Failure with Reduced Ejection Fraction (LVEF ≤ 40%)
- Patients with HFrEF should be treated early with a combination of four medications, one from each of the following classes: angiotensin receptor-neprilysin inhibitor (ARNI), beta-blocker (β-blocker), MRA, and SGLT2 inhibitor (SLGTi). This GDMT/quadruple therapy has been shown to have substantial and sustained reductions in mortality, HF hospitalizations, and symptom burden, even at low doses.5,10–17
- It is preferable to titrate medication doses simultaneously and as quickly as possible (“in-parallel” approach), rather than titrating one medication before initiating an additional agent (“strict sequential” approach).18
- The principles behind treatment are to: treat early, use combination of all four medications, up titrate rapidly, and aim for target dose of each medication.
- Consider IV iron therapy for patients with HFrEF who meet all of the following criteria: ejection fraction ≤ 40%, serum ferritin < 100 μg/L or between 100-299 μg/L, and TSAT < 20%.18
- See Appendix C: Commonly Used Drugs for in Heart Failure Care for dosages, Pharmacare coverage and therapeutic considerations for drug classes used in the management of patients with HF. The appendix also includes titration and renal function considerations. Consider consultation with your local specialist or a RACE line clinician when further guidance is needed.
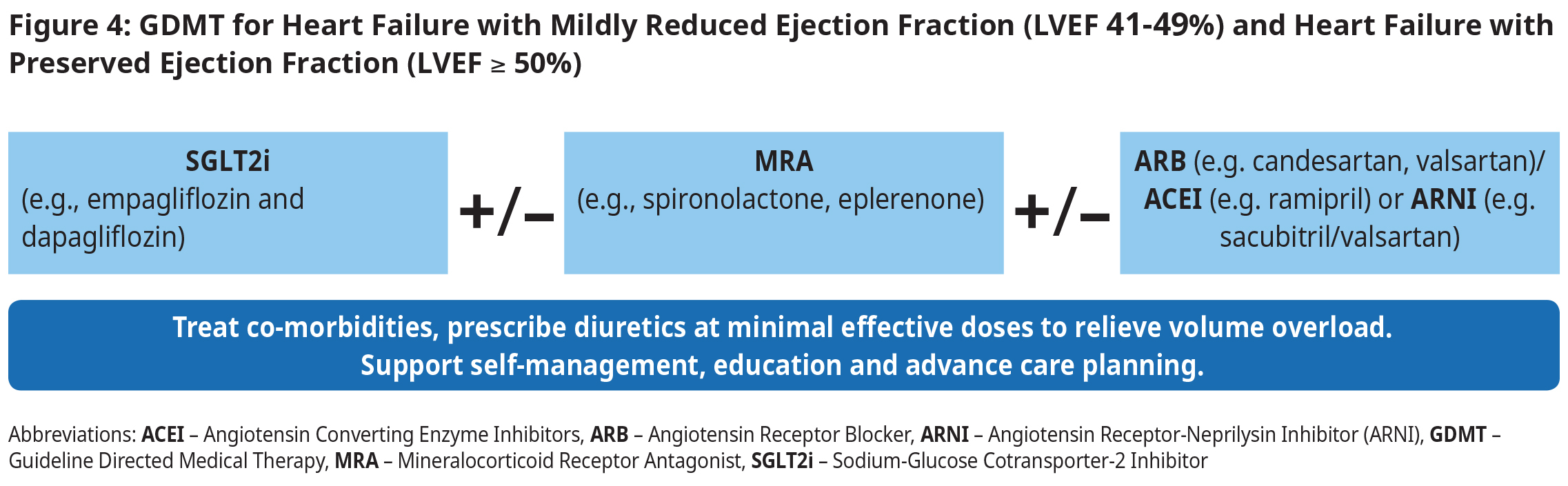
Heart Failure with Mildly Reduced Ejection Fraction (EF 41-49%) and Heart Failure with Preserved Ejection Fraction (EF ≥ 50%)
- To improve symptoms:
- Use minimal effective diuretic dose to achieve and maintain euvolemia.
- To improve outcomes:
- Initiate an SGLT2i (e.g., empagliflozin and dapagliflozin). SGLT2i therapy reduces hospitalizations as well as all- cause and cardiovascular mortality across age, sex, race, HF and EF classification.5,19,20
- Consider MRA (in all eligible patients) to reduce HF hospitalization.21
- In select patients, consider initiating an angiotensin receptor-neprilysin inhibitor (ARNI). In large randomized clinical trials, patients with HFmrEF had fewer hospitalizations when treated with an ARNI compared to an Angiotensin Converting Enzyme Inhibitors (ACEI).
Reassessment and Follow-up
- For all patients, regardless of their EF status, periodic assessment of renal function and potassium is recommended. Renal function and electrolytes should be checked with each adjustment of diuretic, MRA, and/or ACEI/ARB/ARNI dose.
- For patients with HFrEF, EF should be reassessed after maximally tolerated GDMT has been achieved, and before deciding on further treatment options.
- Once clinically optimized, routine surveillance with ECHO is not indicated unless the patient’s clinical status changes. A follow-up ECHO may be warranted in 3-5 years.
Clinical follow-up frequency recommendations:
1-4 weeks or as clinically indicated:
- Those classified with NYHA III or IV symptoms, recent HF hospitalization, during titration of HF medications, new onset heart failure, complications of HF therapy (e.g., rising creatinine, hypotension), needing to down-titrate
- or discontinue ß-blockers or ACEI/ARB, severe concomitant and active illness (e.g., COPD, frailty), frequent implantable cardioverter defibrillator (ICD) firings.
1-6 Months:
- Those with no clear features of high or low risk.
6-12 months:
- NYHA I or II.
- No hospitalizations in past year.
- No recent changes in medications, receiving optimal medical/device HF therapies.
Worsening HF:
- By the time a person reports worsening symptoms of fluid overload or has overt signs of fluid overload on clinical exam, they may have been progressing over days or even weeks. They should be addressed as soon as possible to avoid further decline, hospitalization or even death.
- Identify triggers and treat appropriately. Treat volume overload, optimize therapies and reinforce education.
- If a patient fails to improve, consider referral to a heart failure clinic and/or specialist, including RACE.
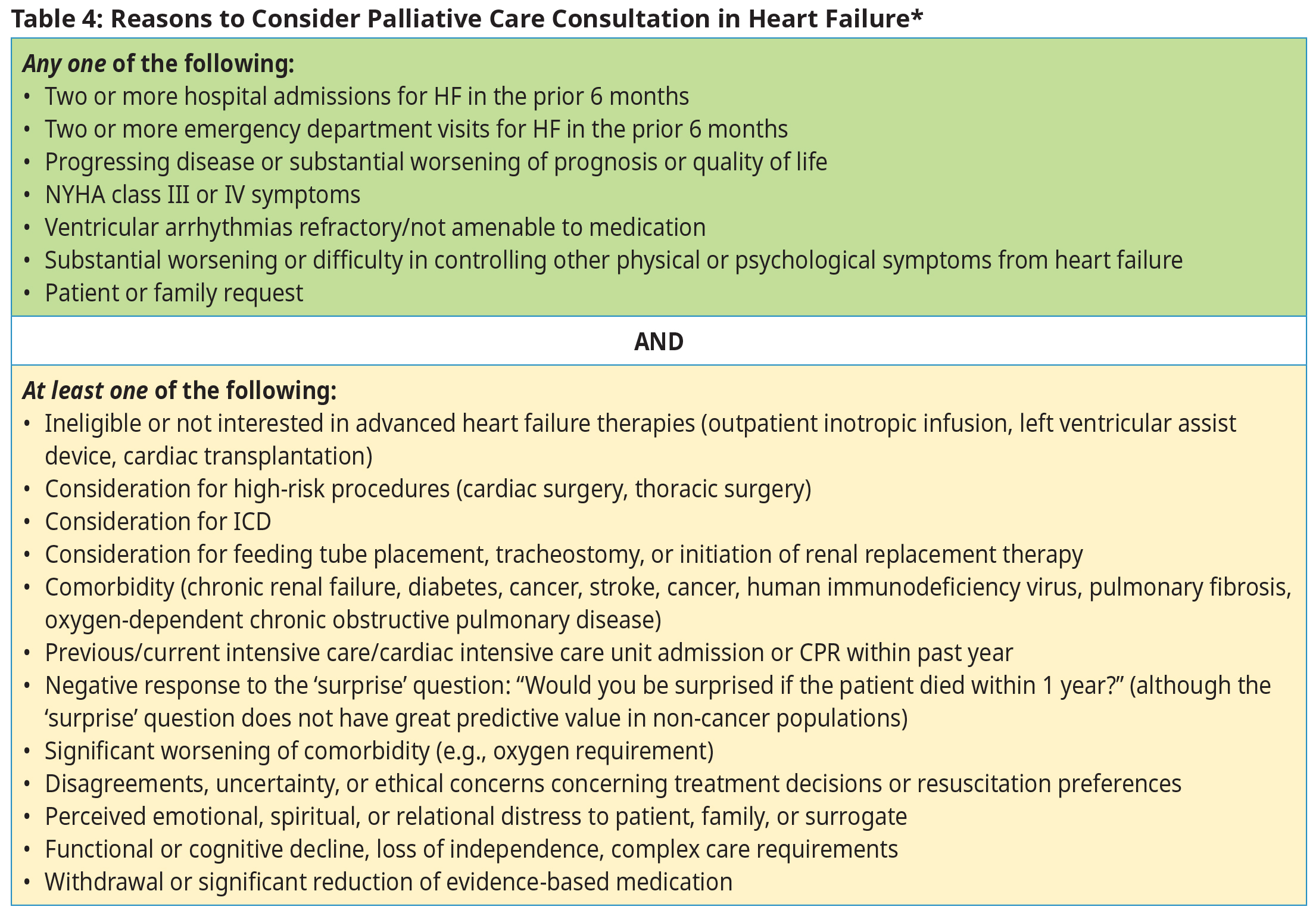
Indications for Referral
Refer to a Cardiologist/Internist:
- Persistent symptoms or signs of HF despite attempts TO OPTIMIZE medical therapy.
- To aid in diagnosis and management of HF where valvular disease or coronary disease is a significant contributing factor.
- If a rare underlying etiology is suspected.
- For assistance with diagnosis or management, refer the patient to a heart function clinic or a multi-disciplinary chronic disease management clinic, if available.
Refer to a Heart Function Clinic, if available, for:
- Management after a recent admission or repeated admissions to hospital.
- Assessment of asymptomatic left ventricular dysfunction.
- Assistance with multidisciplinary HF care including education on lifestyle management skills.
- Consideration for procedural interventions including PCI, surgery, and implantable devices.
- See Cardiac Services BC for Heart Function Clinics in BC
Device Management:
- Decisions about device management (e.g., implantable cardioverter defibrillator [ICD], cardiac resynchronization therapy [CRT]) are complex and involve a cardiologist/internist/cardiac electrophysiologist/Heart Function Clinic.
- Appropriate ICD utilization is associated with improved mortality, but does not improve quality of life. By contrast, CRT is associated with improved mortality and quality of life (i.e., improved symptom status and functional capacity) in properly selected individuals.18
ICD may be considered in patients with one of the following:
- History of hemodynamically significant sustained ventricular arrhythmia;
- Ischemic cardiomyopathy and LVEF ≤ 35% (measured at least 1 month after acute myocardial infarction, or 3 months post coronary artery revascularization); or
- Non-ischemic cardiomyopathy and LVEF ≤ 35% (measured at least 9 months after optimal medical therapy).
- ICD should not be considered in patients with active NYHA Class IV symptoms.
CRT may be considered in patients with NYHA class II – IV symptoms despite maximally tolerated HF therapies, with a QRS duration ≥ 130 ms, and LVEF ≤ 35%.
Palliative Care and End of Life Care
Palliative care offers the potential to improve quality of life for cardiac patients with advanced disease. Unfortunately, many patients perceive palliative care in a negative context, equating it with hospice care. As a result, very few cardiac patients receive appropriate palliative care. For those who do, it typically occurs quite late in the disease trajectory, limiting the benefit. Do not delay advance care planning conversations or palliative care for a patient with serious illness who has physical, psychological, social, or spiritual distress because they are pursuing disease-directed treatment.
A palliative care approach addresses management of pain, dyspnea, other symptoms, and prioritizes quality of life. This may include support for the patient’s family and caregivers.
Some resources include:
- Supportive Cardiology Tool – a clinician tool which focuses on delivery of care from the primary care perspective.
- What Really Matters Now, a comprehensive resource for patients with advanced heart failure.
- BCGuidelines.ca: Palliative Care for the Patient with Incurable Cancer or Advanced Disease.
- HealthLinKBC: Palliative Care and MOST forms
 *Adapted from Slawnych, New Dimensions in Palliative Care Cardiology, CJC, 201822
*Adapted from Slawnych, New Dimensions in Palliative Care Cardiology, CJC, 201822
Resources
References
- Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Journal of Cardiac Failure 2021 Apr. 1;27(4):387–413.
- Roger VL. Epidemiology of Heart Failure. Circulation Research. 2021 May 14;128(10):1421–34.
- Chamberlain AM, Boyd CM, Manemann SM, Dunlay SM, Gerber Y, Killian JM, et al. Risk Factors for Heart Failure in the Community: Differences by Age and Ejection Fraction. Am J Med. 2020 Jun;133(6):e237–48.
- Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Canadian Journal of Cardiology. 2017 Nov;33(11):1342–433.
- 2022 ACC/AHA/HFSA Guideline for the Management of Heart Failure: Executive Summary - ScienceDirect [Internet]. [cited 2022 Jun 8]. Available from: https://www-sciencedirect-com.ezproxy.hlth.gov.bc.ca/science/article/abs/pii/S1071916422000756
- Yu DSF, Li PWC, Li SX, Smith RD, Yue SCS, Yan BPY. Effectiveness and Cost-effectiveness of an Empowerment-Based Self-care Education Program on Health Outcomes Among Patients With Heart Failure: A Randomized Clinical Trial. JAMA Netw Open. 2022 Apr 1;5(4):e225982.
- He Y, Ling Y, Guo W, Li Q, Yu S, Huang H, et al. Prevalence and Prognosis of HFimpEF Developed From Patients With Heart Failure With Reduced Ejection Fraction: Systematic Review and Meta-Analysis. Frontiers in Cardiovascular Medicine [Internet]. 2021 [cited 2023 Apr 18];8. Available from: https://www.frontiersin.org/articles/10.3389/fcvm.2021.757596
- Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. The Lancet. 2019 Jan 5;393(10166):61–73.
- Stolfo D, Sinagra G, Savarese G. Evidence-based Therapy in Older Patients with Heart Failure with Reduced Ejection Fraction. Card Fail Rev. 2022 Apr 28;8:e16/
- Miller RJH, Howlett JG, Fine NM. A Novel Approach to Medical Management of Heart Failure With Reduced Ejection Fraction. Can J Cardiol. 2021 Apr;37(4):632–43.
- Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. The Lancet. 2020 Sep 19;396(10254):819–29.
- Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. The Lancet. 2020 Jul 11;396(10244):121–8.
- Sodium–Glucose Cotransporter-2 Inhibitors in Patients With Heart Failure: A Systematic Review and Meta-analysis: Annals of Internal Medicine: Vol 0, No 0 [Internet]. [cited 2022 May 20]. Available from: https://www.acpjournals.org/doi/10.7326/M21-4284?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
- Cardoso R, Graffunder FP, Ternes CMP, Fernandes A, Rocha AV, Fernandes G, et al. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: A systematic review and meta-analysis. eClinicalMedicine [Internet]. 2021 Jun 1 [cited 2022 Apr 14];36. Available from: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(21)00213-3/fulltext
- McMurray JJV, Packer M. How Should We Sequence the Treatments for Heart Failure and a Reduced Ejection Fraction?: A Redefinition of Evidence-Based Medicine. Circulation. 2021 Mar 2;143(9):875–7.
- Sharma A, Verma S, Bhatt DL, Connelly KA, Swiggum E, Vaduganathan M, et al. Optimizing Foundational Therapies in Patients With HFrEF. JACC: Basic to Translational Science. 2022 Mar;S2452302X21003594.
- Bauersachs J. Heart failure drug treatment: the fantastic four. European Heart Journal. 2021 Feb 7;42(6):681–3.
- McDonald M, Virani S, Chan M, Ducharme A, Ezekowitz JA, Giannetti N, et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Canadian Journal of Cardiology. 2021 Apr 1;37(4):531–46.
- Ghionzoli N, Gentile F, Del Franco AM, Castiglione V, Aimo A, Giannoni A, et al. Current and emerging drug targets in heart failure treatment. Heart Fail Rev. 2022 Jul 1;27(4):1119–36.
- Karamichalakis N, Xanthopoulos A, Triposkiadis F, Paraskevaidis I, Tsougos E. Reshaping Treatment of Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine. 2022 Jan;11(13):3706.
- Kjeldsen SE, von Lueder TG, Smiseth OA, Wachtell K, Mistry N, Westheim AS, et al. Medical Therapies for Heart Failure With Preserved Ejection Fraction. Hypertension. 2020 Jan;75(1):23–32.
- Slawnych M. New Dimensions in Palliative Care Cardiology. Canadian Journal of Cardiology. 2018 Jul 1;34(7):914–24.
Abbreviations:
ACEI Angiotensin-converting enzyme inhibitors
ARNI Angiotensin Receptor-neprilysin Inhibitor
BNP B -Type Natriuretic Peptide
CRT Cardiac Resynchronization Therapy
CVD Cardiovascular Disease
ECG Electrocardiogram
ECHO Echocardiogram
GDMT Guideline Directed Medical Therapy
HF Heart Failure
HFmrEF HF Mild Reduced Ejection Fraction
HFpEF HF Preserved Ejection Fraction
HFrEF HF Reduced Ejection Fraction
ICD Implantable Cardioverter Defibrillator
LVEF Left Ventricular Ejection Fraction
MIBI Thallium and Sestamibi scan
MRA Mineralocorticoid Receptor Antagonist
MUGA Multi Gated Acquisition Scan
NT-proBNP N‐terminal pro B‐type natriuretic peptide
NYHA New York Heart Association
SGLT2i Sodium-Glucose Cotransporter-2 Inhibitor
TSH Thyroid Stimulating Hormone
Practitioner Resources
- Cardiac Services BC, www.cardiacbc.ca generates and shares accurate, current, and relevant HF information for health care professionals and patients in BC.
- RACE: Rapid Access to Consultative Expertise Program – RACE means timely telephone advice from specialist for Physicians, Medical Residents, Nurse Practitioners, Midwives, all in one phone call.
- Monday to Friday 0800 – 1700
- Online at www.raceapp.ca or though Apple or Android mobile device. For more information on how to download RACE mobile applications, please visit www.raceconnect.ca/race-app/
- Local Calls: 604–696–2131 | Toll Free: 1–877–696–2131
- For a complete list of current specialty services visit the Specialty Areas page.
- BC Ministry of Health – Advance Care Planning, HealthLinkBC Planning for Advance Care. Each health authority also has an Advance Care Planning website.
Patient, Family and Caregiver Resources
- CardiacServicesBC (www.cardiacbc.ca): Multiple patient handouts available.
- HealthLinkBC: Heart Failure Overview
- HealthLinkBC: Heart Failure: Taking Medicines Properly
- HealthLinBC: Monitoring and Medicines for Heart Failure
- Island Health Community Virtual Care: Community Virtual Care provides support to people with a range of medical conditions. Registered nurses help you to manage your condition from the comfort of your home. All the tools needed are loaned to you at no cost.
- Canadian Heart Failure Society: Living Well with Heart Failure
- Canadian Heart Failure Society: How to manage your heart failure medication ON SICK DAYS?
- RxFiles: SADMANS medications in heart failure
- Heartlife Foundation: Patients
- Heart Failure Medications: A Patient and Caregiver Guide
- Heart and Stroke Foundation: Heart Failure
Diagnostic Fee Code
428 Heart Failure
Appendices
- Appendix A: Health Behavior Modifications and Self-monitoring Recommendations
- Appendix B: Heart Failure Zones Reference Guide
- Appendix C: Commonly used Drugs in Heart Failure Care
Associated Documents
The following documents accompany this guideline:
- Heart Function Clinic Referral Form
- What to Do with Heart Failure Medications When I am Sick?
- List of Contributors
This guideline is based on scientific evidence current as of the Effective Date.
This guideline was developed by the Guidelines and Protocols Advisory Committee in collaboration with the Provincial Laboratory Medicine Services and adopted under the Medical Services Act and the Laboratory Services Act.
The principles of the Guidelines and Protocols Advisory Committee are to:
Contact InformationGuidelines and Protocols Advisory Committee PO E-mail: hlth.guidelines@gov.bc.ca Web site: www.BCGuidelines.ca Disclaimer The Clinical Practice Guidelines (the guidelines) have been developed by the guidelines and Protocols Advisory Committee on behalf of the Medical Services Commission. The guidelines are intended to give an understanding of a clinical problem, and outline one or more preferred approaches to the investigation and management of the problem. The guidelines are not intended as a substitute for the advice or professional judgment of a health care professional, nor are they intended to be the only approach to the management of clinical problem. We cannot respond to patients or patient advocates requesting advice on issues related to medical conditions. If you need medical advice, please contact a health care professional. |